The role of the COVID-19 infection in autonomic disorders
During the year of the pandemic, morbidity and mortality statistics increased significantly. Acute cardiovascular diseases and suicides are at the forefront [1–5], and there are still no approved antiviral drugs or effective long-term vaccines to control coronavirus disease 2019 (COVID-19). However, to address this problem around the world, to counter the pandemic, serious efforts have been made [6–9].
This required the genomic characterization of a new human pathogenic coronavirus isolated from a patient with severe acute respiratory syndrome after visiting Wuhan in 2019 [10, 11]. After that, deep metagenomic sequencing and polymerase chain reaction have become fundamental for studying infection of a severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) and the mechanisms of its mutation, as well as the creation of drugs based on genetic engineering [12–16].
In a healthy person, the stability of the internal environment tends to homeostasis. Homeostasis is not a result but a goal of the whole organism. All organs and systems aim at this [17, 18].
The disease leads to a shift in homeostasis, which is named allostasis in the concepts of integrative physiology. An example of homeostasis can be normotonia of the autonomic nervous system (ANS) (Fig. 1a). Allostasis is a shift of curves for oppositely operating effectors of sympathetic (SNS) and parasympathetic nervous systems (PNS) that leads to regulation of the changeable variable autonomic nervous system at other levels (Fig. 1b).
Purpose: to analyze the pathogenetic features of the autonomic nervous system lesion by coronavirus infection to identify patterns that could predict the disease course and improve treatment.
Evolution of views on stress management
In the process of regulating homeostasis, the main role belongs to neurohumoral regulation, which determines the response to stress. For the first time, stress conceptualization as a non-specific organism reaction to an external stimulus was proposed by Hans Selye as a “general adaptation syndrome” (1950). It included stages such as anxiety, resistance and exhaustion [19, 20].
In the 1990s after the proposed theory of the central stress system existence, which would cause activation of a stress syndrome, George Chrousos and Philip Gold of the US National Institutes of Health were the first who proposed the concept of integration of the autonomic nervous system and humoral mechanisms in the regulation of the central stress system components (Fig. 2) [21, 22].
In the short term, activation of this system can greatly shift homeostasis toward allostasis, which can be important for survival in critical situations, without consequences for the body. Long-term activation of ANS in cases that do not threaten human life will cause damage, depleting the body resources [23, 24].
As the preconditions for understanding the problem of autonomic disorders appeared in the early stages of treating patients with COVID-19, researchers began to worry about a more detailed study of what is traditionally named the autonomic nervous system, consisting of parasympathetic nervous system, sympathetic nervous system and intestinal nervous system. Increasing the meaning of the term “autonomic”, they brought to the fore neuroendocrine and neuroimmune systems [22, 23]. Thus, researchers introduced the term “extended autonomic system” (EAS) using some integrative concepts of physiology — homeostasis, allostasis and stress based on which the main assumption was made about the existence of biomarkers of EAS activation [23] (Fig. 3).
Ways of coronavirus infection migration
Numerous researchers suggest that such a massive spread of SARS-CoV-2 and severe autonomic nervous system disorders may be due to its high neurotropism. During the review of various independent scientific studies, two groups of patients were identified depending on the neurological and pathogenetic features [25–31].
The first group consisted of patients in whom SARS-CoV-2 migrated into the brain through the blood pene-trating the blood-brain barrier [30–36]. The generali-zation of the results in this group correlates with SARS-CoV-2 effect on the renin-angiotensin system (RAS) through angiotensin-converting enzyme [37, 40]. Under these conditions, there is often a positive feedback loop between RAS activation and tonic enhancement of efferent sympathetic nerve activity. Increased sympathetic activity may stimulate RAS activation, which may further stimulate sympathetic activity (Díaz et al., 2020; Guan et al., 2020; Hoffmann et al., 2020).
The second group included patients with the parasympathetic central nervous system disorders due to possible increased neurotropism, mainly of the vagus nerve and the olfactory bulb. This correlates with the data from other works, which put forward a different route of coronavirus infection migration, directly through the nerve trunks of the vagus nerve and the olfactory bulb [41–44].
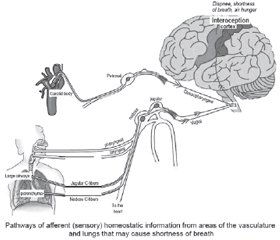
Normally, when stimulating chemoreceptors and mechanoreceptors of these nerves, signals are transmitted to the brain stem through the glossopharyngeal nerve and vagus nerve, converging in the NTS. Then signals go to the somatosensory cortex and other higher brain centers, which provide interoception of the internal environment of the body. The processing of these signals in the cerebral cortex causes feelings such as hunger, shortness of breath, or dyspnea [44–48].
Patients with coronavirus infection are likely to have the inhibition of processing these signals.
A randomized, blind, placebo-controlled study of healthy people with non-invasive transcutaneous vagus nerve stimulation indicates a decreasing release of inflammatory cytokines, which provides an anti-inflammatory effect [46–49].
Thus, the pathogenesis of COVID-19 and the proposed theories of SARS-CoV-2 penetration into the brain can be imagined as a homeostasis shift towards the sympathetic nervous system (Fig. 4) where the SNS and PNS disorders are observed, but pathogenetic links and severity of violations are still not known.
Conclusions
1. The study of the autonomic nervous system disorders confirms the proposed theories of the two main routes of coronavirus infection migration.
2. The proposed new approaches to the correction of autonomic disorders in patients with COVID-19 using non-invasive transcutaneous stimulation need to be correlated with the RAS system study.
3. The results of independent studies confirm the possible neurotropism of the virus by two pathways of migration to the central structures of the brain.
The prospect of further study of ANS disorders in patients with coronavirus infection is to identify the priority routes of coronavirus infection migration, as well as the severity of damage to the autonomic nervous system, which would make it possible to predict and improve treatment outcomes compared to standard methods of treating patients with COVID-19.
Received 28.10.2021
Revised 10.11.2021
Accepted 15.11.2021
Список литературы
1. Lucas Böttcher, Maria R. D’Orsogna, Tom Chou. Using excess deaths and testing statistics to improve estimates of COVID-19 mortalities. medRxiv. 2021. Preprint. doi: 10.1101/2021.01.10.21249524.
2. Andrea Ganna. Mapping the human genetic architecture of COVID-19 by worldwide meta-analysis. The COVID-19 Host Genetics Initiative. medRxiv. 2021. doi: 10.1101/2021.03.10.21252820.
3. Bismark Singh. International comparisons of COVID-19 deaths in the presence of comorbidities require uniform mortality coding guidelines. International Journal of Epidemiology. 2021. 50(2). 373-377. doi: 10.1093/ije/dyaa276.
4. Mike K.P. So, Amanda M.Y. Chu, Agnes Tiwari, Jacky N.L. Chan. On topological properties of COVID-19: predicting and assessing pandemic risk with network statistics. SoSci. Rep. 2021. 11. 5112. doi: 10.1038/s41598-021-84094-z.
5. Офіційний сайт Всесвітньої організації охорони здоров’я. Оперативна статистична інформація [Електронний ресурс]. Режим доступу: http://www.who.int/.
6. David Hillus, Tatjana Schwarz, Pinkus Tober-Lau, Kanika Vanshylla, Hana Hastor, Charlotte Thibeault et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir. Med. 2021. doi: 10.1016/S2213-2600(21)00357-X.
7. Pinkus Tober-Lau, Tatjana Schwarz, Kanika Vanshylla, David Hillus, Henning Gruell et al., the EICOV/COVIM Study Group. Long-term immunogenicity of BNT162b2 vaccination in older people and younger health-care workers. Lancet Respir. Med. 2021. 9(11). E104-E105. doi: 10.1016/S2213-2600(21)00456-2.
8. Gregory Milne, Thomas Hames, Chris Scotton, Nick Gent, Ale-xander Johnsen, Roy M. Anderson et al. Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity? Lancet Respir. Med. 2021. doi: 10.1016/S2213-2600(21)00407-0.
9. Intikhab Alam, Allan Kamau, Maxat Kulmanov, Stefan T. Arold, Takashi Gojobori, Arnab Pain, Carlos M. Duarte. Functional pangenome analysis provides insights into the origin, function and pathways to therapy of SARS-CoV-2 coronavirus. bioRxiv. 2020. doi: 10.1101/2020.02.17.952895.
10. Jasper Fuk-Woo Chan, Kin-Hang Kok, Zheng Zhu, Hin Chu, Kelvin Kai-Wang To, Shuofeng Yuan et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020. 9(1). 221-236. doi: 10.1080/22221751.2020.1719902.
11. Emily C.W. Hung, Stephen S.C. Chim, Paul K.S. Chan, Yu K. Tong, Enders K.O. Ng, Rossa W.K. et al. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin. Chem. 2003. 49(12). 2108-2109. doi: 10.1373/clinchem.2003.025437.
12. Caner Bagci, David Bryant, Banu Cetinkaya, Daniel H. Huson. Microbial phylogenetic context using phylogenetic outlines. Genome Biology and Evolution. 2021. 13(9). doi: 10.1093/gbe/evab213.
13. Jian-Yu Jiao, Lan Liu, Zheng-Shuang Hua, Bao-Zhu Fang, En-Min Zhou, Nimaichand Salam et al. Microbial dark matter coming to light: challenges and opportunities. National Science Review. 2021. 8(3). doi: 10.1093/nsr/nwaa280.
14. Рекомендації ВООЗ щодо діагностування COVID-19. Режим доступу: https://www.who.int/ru/news/item/29-01-2021-who-publishes-new-essential-diagnostics-list-and-urges-countries-to-prioritize-investments-in-testing
15. ВОЗ: геномное секвенирование SARS-CoV-2 для целей общественного здравоохранения. Режим доступа: https://apps.who.int/iris/bitstream/handle/10665/338483/WHO-2019-nCoV-genomic_sequencing-2021.1-rus.pdf
16. Selye H. Stress without distress. New American Library. New York, 1974. 193.
17. Nicolaides N.C., Kyratzi E., Lamprokostopoulou A., Chrousos G.P., Charmandari E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation. 2015. 22(1–2). 6-19. doi: 10.1159/000362736.
18. Cantor D., Ramsden E. Stress, shock, and adaptation in the twentieth century. Rochester (NY): University of Rochester Press, 2014. https://www.ncbi.nlm.nih.gov/books/NBK189532/?report=classic
19. Sternberg E.M., Chrousos G.P., Wilder R.L. The stress response and the regulation of inflammatory disease. Ann. Intern. Med. 1992. 117. 854-866.
20. Agorastos Agorastos, Nicolaides Nicolas C., Bozikas Vasilios P., Chrousos George P., Panagiota Pervanidou. Multilevel interactions of stress and circadian system: implications for traumatic stress. Journal Frontiers in Psychiatry. 2020. 10. 1003. doi: 10.3389/fpsyt.2019.01003.
21. Chrousos G.P., Gold P.W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. J. Am. Med. Assoc. 1992. 267. 1244-1252.
22. Chigr F., Rachidi F., Tardivel C. Modulation of orexigenic and anorexigenic peptides gene expression in the rat DVC and hypothalamus by acute immobilization stress. Front. Cell. Neurosci. 2014. 8. 198. doi: 10.3389/fncel.2014.00198.
23. Goldstein D.S. The extended autonomic system, dyshomeostasis, and COVID-19. Clin. Auton. Res. 2020. 30. 299-315. doi: 10.1007/s10286-020-00714-0.
24. Elizabeth O. Johnson, Themis C. Kamilaris, George P. Chrousos, Philip W. Gold. Mechanisms of stress: A dynamic overview of hormonal and behavioral homeostasis. Neuroscience & Biobehavioral Reviews. 1992. 6(2). 115-130. doi: 10.1016/S0149-7634(05)80175-7.
25. Yeshun Wu, Xiaolin Xu, Zijun Chen, Jiahao Duan, Kenji Hashimoto, Ling Yang et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Behavior and Immunity. 2020. 87. 18-22. doi: 10.1016/j.bbi.2020.03.031.
26. Filatov A., Sharma P., Hindi F., Espinosa P.S. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020. 12(3). e7352. doi: 10.7759/cureus.7352.
27. Del Rio Rodrigo, Marcus Noah J., Inestrosa Nibaldo C. Potential role of autonomic dysfunction in COVID-19 morbidity and mortality. Frontiers in Physiology. 2020. 11. 1248. doi: 10.3389/fphys.2020.561749.
28. Peter W. Abel, Michael T. Piascik. Introduction to autonomic nervous system drugs. Pharmacology and Therapeutics for Dentistry. 7th ed. 2017. 71-81. doi: 10.1016/B978-0-323-39307-2.00005-9.
29. Ling Mao, Huijuan Jin, Mengdie Wang, Yu Hu, Shengcai Chen, Quanwei He et al. Neurologic manifestations of hospitalized patients 2020 with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020. 77(6). 683-690. doi: 10.1001/jamaneurol.2020.1127.
30. Isaac H. Solomon, Erica Normandin, Shamik Bhattacharyya, Shibani S. Mukerji, Ahya S. Ali, Gordon Adams et al. Neuropathological features of COVID-19. N. Engl. J. Med. 2020. 383. 989-992. doi: 10.1056/NEJMc2019373.
31. González-Duarte A., Norcliffe-Kaufmann L. Is “happy hypoxia” in COVID-19 a disorder of autonomic interoception? A hypothesis. Clin. Auton Res. 2020. 30. 331-333. doi: 10.1007/s10286-020-00715-z.
32. Consuelo Gutiérrez-Ortiz, Antonio Méndez-Guerrero, Sara Rodrigo-Rey, Eduardo San Pedro-Murillo, Laura Bermejo-Guerrero, Ricardo Gordo-Mañas et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020. 95(5). doi: 10.1212/WNL.0000000000009619.
33. Sharifian-Dorche M., Huot P., Osherov M., Wen D., Saveriano A., Giacomini P.S. et al. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. Neurol. Sci. 2020. 417(117085). doi: 10.1016/j.jns.2020.117085.
34. Toovey S. Influenza-associated central nervous system dysfunction: a literature review. Travel Med. Infect. Dis. 2008. 6(3). 114-24. doi: 10.1016/j.tmaid.2008.03.003.
35. Yeshun Wu, Xiaolin Xu, Zijun Chen, Jiahao Duan, Kenji Hashimoto, Ling Yang et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain, Behavior and Immunity. 2020. 87. 18-22. doi: 10.1016/j.bbi.2020.03.031.
36. Nanshan Chen, Min Zhou, Xuan Dong, Jieming Qu, Fengyun Gong, Yang Han et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020. 395(10223). 507-13. doi: 10.1016/S0140-6736(20)30211-7.
37. Ghazal Aghagoli, Benjamin Gallo Marin, Nicole J. Katchur, Franz Chaves-Sell, Wael F. Asaad, Sarah A. Murphy. Neurological involvement in COVID-19 and potential mechanisms: a review. Neurocrit. Care. 2021. 34. 1062-1071. doi: 10.1007/s12028-020-01049-4.
38. Fatiha Chigr, Mohamed Merzouki, Mohamed Najimi. Autonomic brain centers and pathophysiology of COVID-19. ACS Chemical Neuroscience. 2020. 11(11). 1520-1522. doi: 10.1021/acschemneuro.0c00265.
39. Melanie Dani, Andreas Dirksen, Patricia Taraborrelli, Miriam Torocastro, Dimitrios Panagopoulos, Richard Sutton et al. Autonomic dysfunction in “long COVID”: rationale, physiology and management strategies. Clin. Med. 2021. 21(1). e63-67. doi: 10.7861/clinmed.2020-0896.
40. Sata Yusuke, Head Geoffrey A., Denton Kate, May Clive N., Schlaich Markus P.J. Role of the sympathetic nervous system and its modulation in renal hypertension. Frontiers in Medicine. 2018. 5. 82. doi: 10.3389/fmed.2018.00082.
41. Gustavo C. Román, Peter S. Spencer, Jacques Reis, Alain Buguet, Mostafa El Alaoui Faris, Sarosh M. et al. The neurology of COVID-19 revisited: a proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. Journal of the Neurological Sciences. 2020. 414(116884). doi: 10.1016/j.jns.2020.116884.
42. Jerome R. Lechien, Carlos M. Chiesa-Estomba, Daniele R. De Siati, Mihaela Horoi, Serge D. Le Bon, Alexandra Rodriguez et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 2020. 277. 2251-2261 doi: 10.1007/s00405-020-05965-1.
43. Stuart J. McDougall, Michael C. Andresen. Independent transmission of convergent visceral primary afferents in the solitary tract nucleus. J. Neurophysiol. 2013. 109(2). 507-517. doi: 10.1152/jn.00726.2012.
44. Alberto Paniz-Mondolfi, Clare Bryce, Zachary Grimes, Ronald E. Gordon, Jason Reidy, John Lednicky et al. Central nervous system involvement by severe respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Med. Virol. 2020. 92(7). 699-702. doi: 10.1002/jmv.25915.
45. Martin C.R., Osadchiy V., Kalani A., Mayer E.A. The brain-gut-microbiome axis. Cellular and Molecular Gastroenterology and Hepatology. 2018. 6(2). 133-48. doi: 10.1016/j.jcmgh.2018.04.003.
46. Bonaz B., Sinniger V., Pellissier S. Vagal tone: effects on sensitivity, motility, and inflammation. Neurogastroenterology & Motility. 2016. 28(4). 455-62. doi: 10.1111/nmo.12817.
47. Imanuel Lerman, Richard Hauger, Linda Sorkin, James Proudfoot, Bryan Davis, Andy Huang et al. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: a randomized, blinded, healthy control pilot trial. Neuromodulator. 2016. 19. 283-290. doi: 10.1111/ner.12398.
48. Breit Sigrid, Kupferberg Aleksandra, Rogler Gerhard, Hasler Gregor. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. J. Frontiers in Psychiatry. 2018. 9. 44. doi: 10.3389/fpsyt.2018.00044.
49. Hočevar A., Tomšič M., Praprotnik S., Hojnik M., Kveder T., Rozman B. Parasympathetic nervous system dysfunction in primary Sjögren’s syndrome. Electronic Journal Article. 2003. 62(8). 702-704. doi: 10.1136/ard.62.8.702.
/73_2.jpg)
/74_2.jpg)

/73.jpg)
/74.jpg)