Журнал «Здоровье ребенка» Том 16, №8, 2021
Вернуться к номеру
Сучасні рекомендації щодо лікування атопічного дерматиту й харчової алергії в дітей
Авторы: Няньковський С.Л., Няньковська О.С., Яцула М.С., Городиловська М.І.
Львівський національний медичний університет імені Данила Галицького, м. Львів, Україна
Рубрики: Педиатрия/Неонатология
Разделы: Справочник специалиста
Версия для печати
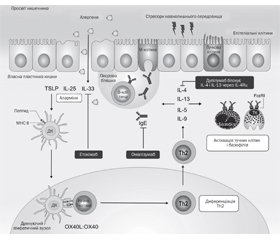
Атопічний дерматит є найпоширенішим хронічним дитячим запальним захворюванням шкіри. Хронічний і рецидивуючий характер атопічного дерматиту вимагає як активної терапії загострень, так і підтримуючої терапії для підвищення цілісності шкірного бар’єра й запобігання майбутнім рецидивам. Системна терапія є виправданою для пацієнтів, які мають поганий контроль над захворюванням (тяжкий перебіг або значний вплив на якість життя), незважаючи на відповідну місцеву терапію і/або фототерапію. За останні кілька десятиліть поширеність харчової алергії продовжує зростати. Від харчової алергії страждає 6–13 % населення планети. Існує 2 основних типи ліків, які можна використовувати для полегшення симптомів алергічної реакції на їжу: антигістамінні препарати й адреналін. На амбулаторному етапі для профілактики й лікування реакцій на продукти харчування можуть бути використані антигістамінні середники, зокрема диметиндену малеат — Едермік.
Atopic dermatitis is the most common chronic childhood inflammatory skin disease. The chronic and recurrent nature of pediatric atopic dermatitis requires the use of active therapy for flares and maintenance therapy to promote the integrity of the skin barrier and prevent future flares. Systemic therapy is warranted for patients who have inadequate disease control (persistent severity and extent or significant impact on the quality of life) despite appropriate treatment with topical therapy and/or phototherapy. Over the past several decades, the prevalence of food allergy has continued to increase. It has become a significant health burden affecting 6–13% of the global population. There are two main types of medications that can be used to relieve the symptoms of the allergic reaction to food: antihistamines and adrenaline. Antihistamines can be used for food allergy prevention and treatment, in particular dimetindene maleate — Edermik.
атопічний дерматит; харчова алергія; діти; лікування; диметиндену малеат; Едермік
atopic dermatitis; food allergy; children; treatment; dimetindene maleate; Edermik
Нефармакологічні методи лікування
Фармакологічна терапія
Нові методи лікування
Висновки
- Williams H.C. Clinical practice. Atopic dermatitis. N. Engl. J. Med. 2005. Vol. 352. P. 2314-2324.
- Illi S., von Mutius E., Lau S. et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J. Allergy Clin. Immunol. 2004. Vol. 113. P. 925-931.
- Sidbury R., Davis D.M., Cohen D.E. et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J. Am. Acad. Dermatol. 2014. Vol. 71(2). P. 327-349. doi: 10.1016/j.jaad.2014.03.030.
- Lansang P., Lam J.M., Marcoux D. et al. Approach to the Assessment and Management of Pediatric Patients With Atopic Dermatitis: A Consensus Document. Section III: Treatment Options for Pediatric Atopic Dermatitis. Journal of Cutaneous Medicine and Surgery. 2019. Vol. 23. P. 19S-31S.
- Boulos S., Yan A.C. Current concepts in the prevention of atopic dermatitis. Clin. Dermatol. 2018. Vol. 36(5). P. 668-671. doi: 10.1016/j.clindermatol.2017.03.004.
- Angelova-Fischer I., Neufang G., Jung K. et al. A randomized, investigator-blinded efficacy assessment study of stand-alone emollient use in mild to moderately severe atopic dermatitis flares. J. Eur. Acad. Dermatol. Venereol. 2014. Vol. 28. P. 9-15. doi: 10.1111/jdv.12479.
- Horimukai K., Morita K., Narita M. et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J. Allergy Clin. Immunol. 2014. Vol. 134(4). P. 824-830. doi: 10.1016/j.jaci.2014.07.060.
- Xu S., Immaneni S., Hazen G.B. et al. Cost-effectiveness of prophylactic moisturization for atopic dermatitis. JAMA Pediatr. 2017. Vol. 171(2). P. e163909. doi: 10.1001/jamapediatrics.2016.3909.
- van Zuuren E.J., Fedorowicz Z., Christensen R. et al. Emollients and moisturisers for eczema. Cochrane Database Syst. Rev. 2017. Vol. 2017(2). P. CD012119.
- Brar K.K., Nicol N.H., Boguniewicz M. Strategies for successful management of severe atopic dermatitis. J. Allergy Clin. Immunol. 2019. Vol. 7(1). P. 1-16. doi: 10.1016/j.jaip.2018.10.021.
- Eichenfield L.F., Ahluwalia J., Waldman A. et al. Current guidelines for the evaluation and management of atopic dermatitis: acomparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J. Allergy Clin. Immunol. 2017. Vol. 139(4S). P. S49-S57. doi: 10.1016/j.jaci.2017.01.009.
- Cardona I.D., Stillman L., Jain N. Does bathing frequency matter in pediatric atopic dermatitis? Ann. Allergy, Asthma Immunol. 2016. Vol. 117(1). P. 9-13. doi: 10.1016/j.anai.2016.05.014.
- Santer M., Ridd M.J., Francis N.A. et al. Emollient Bath additives for the treatment of childhood eczema (BATHE): multicentre pragmatic parallel group randomised controlled trial of clinical and cost effectiveness. BMJ. 2018. Vol. 361(8151). P. k1332.doi: 10.1136/bmj.k1332.
- Katoh N., Ohya Y., Ikeda М. et al. Japanese guidelines for atopic dermatitis 2020. Allergology International. 2020. Vol. 69. P. 356e-369.
- Assarian Z., O’Brien T.J., Nixon R. Soak and smear: an effective treatment for eczematous dermatoses. Australas J. Dermatol. 2015. Vol. 56(3). P. 215-217. doi: 10.1111/ajd.12300.
- Chopra R., Vakharia P.P., Sacotte R., Silverberg J.I. Efficacy of bleach baths in reducing severity of atopic dermatitis: a systematic review and meta-analysis. Ann. Allergy, Asthma Immunol. 2017. Vol. 119(5). P. 435-440. doi: 10.1016/j.anai.2017.08.289.
- González-López G., Ceballos-Rodríguez R.M., González-López J.J. et al. Efficacy and safety of wet wrap therapy for patients with atopic dermatitis: a systematic review and meta-analysis. Br. J. Dermatol. 2017. Vol. 177(3). P. 688-695. doi: 10.1111/bjd.15165.
- Lim N.R., Lohman M.E., Lio P.A. The role of elimination diets in atopic Dermatitis-A comprehensive review. Pediatr. Dermatol. 2017. Vol. 34(5). P. 516-527. doi: 10.1111/pde.13244.
- Bath-Hextall F., Delamere F.M., Williams H.C. Dietary exclusions for improving established atopic eczema in adults and children: systematic review. Allergy Eur. J. Allergy Clin. Immunol. 2009. Vol. 64(2). P. 258-264. doi: 10.1111/j.1398-9995.2008.01917.x.
- Wollenberg A., Barbarot S., Bieber T. et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. JEADV. 2018. 32. 657-682. DOI: 10.1111/jdv.14891.
- Shelley A.J., McDonald K.A., McEvoy A. et al. Usability, satisfaction, and usefulness of an illustrated eczema action plan. J. Cutan. Med. Surg. 2018. Vol. 22(6). P. 577-582. doi: 10.1177/1203475418789028.
- Schwartz P.J., Woosley R.L. Predicting the unpredictable: drug-induced QT prolongation and torsades de pointes. J. Am. Coll. Cardiol. 2016. Vol. 67(13). P. 1639-1650. doi: 10.1016/j.jacc.2015.12.063.
- Eichenfield L.F., Tom W.L., Berger T.G. et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J. Am. Acad. Dermatol. 2014. Vol. 71(1). P. 116-132. doi: 10.1016/j.jaad.2014.03.023.
- Glines K.R., Stiff K.M., Freeze M. et al. An update on the topical and oral therapy options for treating pediatric atopic dermatitis. Expert Opin. Pharmacother. 2019. Vol. 20(5). P. 621-629. doi: 10.1080/14656566.2018.1561868.
- Wollenberg A., Barbarot S., Bieber T. et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. JEADV. 2018. Vol. 32. P. 850-878. https://doi.org/10.1111/jdv.14888.
- Няньковський С.Л., Няньковська О.С., Городиловська М.І. Атопічний дерматит — актуальна проблема сучасної педіатрії. Здоров’я дитини. 2019. Т. 14. С. 250-255. DOI: 10.22141/2224-0551.14.4.2019.174039.
- Mayba J.N., Gooderham M.J. Review of atopic dermatitis and topical therapies. J. Cutan. Med. Surg. 2017. Vol. 21(3). P. 227-236. doi: 10.1177/1203475416685077.
- Sigurgeirsson B., Boznanski A., Todd G. et al. Safety and efficacy of pimecrolimus in atopic dermatitis: a 5-year randomized trial. Pediatrics. 2015. Vol. 135(4). P. 597-606. doi: 10.1542/peds.2014-1990.
- Liu L., Ong G. A randomized, open-label study to evaluate an intermittent dosing regimen of fluticasone propionate 0.05% cream in combination with regular emollient skin care in reducing the risk of relapse in pediatric patients with stabilized atopic dermatitis. J. Dermatolog. Treat. 2018. Vol. 29(5). P. 501-509. doi: 10.1080/09546634.2017.1401211.
- Fukuie T., Hirakawa S., Narita M. et al. Potential preventive effects of proactive therapy on sensitization in moderate to severe childhood atopic dermatitis: a randomized, investigator-blinded, controlled study. J. Dermatol. 2016. Vol. 43(11). P. 1283-1292. doi: 10.1111/1346-8138.13408.
- Huang A., Cho C., Leung D.Y.M., Brar K. Atopic dermatitis: early treatment in children. Curr. Treat. Options Allergy. 2017. Vol. 4(3). P. 355-369. doi: 10.1007/s40521-017-0140-6.
- Callen J., Chamlin S., Eichenfield L.F. et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br. J. Dermatol. 2007. Vol. 156(2). P. 203-221. doi: 10.1111/j.1365-2133.2006.07538.x.
- Moret L., Anthoine E., Aubert-Wastiaux H. et al. TOPICOP©: a new scale evaluating topical corticosteroid phobia among atopic dermatitis outpatients and their parents. PLoS One. 2013. Vol. 8(10). P. e76493. doi: 10.1371/journal.pone.0076493.
- Hon K.L., Leong K.F., Leung T.N.H., Leung A.K.C. Dismissing the fallacies of childhood eczema management: case scenarios and an overview of best practices. Drugs Context. 2018. Vol. 7. P. 1-12. doi: 10.7573/dic.212547.
- Remitz A., De Pità O., Mota A. et al. Position statement: topical calcineurin inhibitors in atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2018. Vol. 32(12). P. 2074-2082. doi: 10.1111/jdv.15272.
- Huang X., Xu B. Efficacy and safety of tacrolimus versus pimecrolimus for the treatment of atopic dermatitis in children: a network meta-analysis. Dermatology. 2015. Vol. 231(1). P. 41-49. doi: 10.1159/000381948.
- Siegfried E.C., Jaworski J.C., Kaiser J.D., Hebert A.A. Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr. 2016. Vol. 16(1). P. 75. doi: 10.1186/s12887-016-0607-9.
- Lynde C.W., Bergman J., Fiorillo L. et al. Clinical insights about topical treatment of mild-to-moderate pediatric and adult atopic dermatitis. J. Cutan. Med. Surg. 2019. Vol. 23 (Suppl. 3). P. 3S-13.doi: 10.1177/1203475419843108.
- Eichenfield L.F., Call R.S., Forsha D.W. et al. Long-term safety of crisaborole ointment 2% in children and adults with mild to moderate atopic dermatitis. J. Am. Acad. Dermatol. 2017. Vol. 77(4). P. 641-649. doi: 10.1016/j.jaad.2017.06.010.
- Mok Z.-R., Koh M.J.-A., Chong W.-S. Is phototherapy useful in the treatment of atopic dermatitis in Asian children? A 5-year report from Singapore. Pediatr. Dermatol. 2014. Vol. 31(6). P. 698-702. doi: 10.1111/pde.12405.
- Dayal S., Pathak K., Sahu P., Jain V.K. Narrowband UV-B phototherapy in childhood atopic dermatitis: efficacy and safety. An. Bras. Dermatol. 2017. Vol. 92(6). P. 801-806. doi: 10.1590/abd1806-4841.20175958.
- Darné S., Leech S.N., Taylor A.E.M. Narrowband ultraviolet B phototherapy in children with moderate-to-severe eczema: a comparative cohort study. Br. J. Dermatol. 2014. Vol. 170(1). P. 150-156. doi: 10.1111/bjd.12580.
- Crall C.S., Rork J.F., Delano S., Huang J.T. Phototherapy in children: considerations and indications. Clin. Dermatol. 2016. Vol. 34(5). P. 633-639. doi: 10.1016/j.clindermatol.2016.05.018.
- Proudfoot L.E., Powell A.M., Ayis S. et al. The European treatment of severe atopic eczema in children taskforce (TREAT) survey. Br. J. Dermatol. 2013. Vol. 169(4). P. 901-909. doi: 10.1111/bjd.12505.
- Totri C.R., Eichenfield L.F., Logan K. et al. Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J. Am. Acad. Dermatol. 2017. Vol. 76(2). P. 281-285. doi: 10.1016/j.jaad.2016.09.021.
- El-Khalawany MA., Hassan H., Shaaban D., Ghonaim N., Eassa B. Methotrexate versus cyclosporine in the treatment of severe atopic dermatitis in children: a multicenter experience from Egypt. Eur. J. Pediatr. 2013. Vol. 172(3). P. 351-356. doi: 10.1007/s00431-012-1893-3.
- Colmenero C.G., Morente G.B., Sánchez J.T. Oral cyclosporine weekend therapy: a new maintenance therapeutic option in patients with severe atopic dermatitis. Pediatr. Dermatol. 2015. 32(4). 551-552. doi: 10.1111/pde.12592.
- Sibbald C., Pope E., Ho N., Weinstein M. Retrospective review of relapse after systemic cyclosporine in children with atopic dermatitis. Pediatr. Dermatol. 2015. 32(1). 36-40. doi: 10.1111/pde.12367.
- Hernández-Martín A., Noguera-Morel L., Bernardino-Cuesta B. et al. Cyclosporine A for severe atopic dermatitis in children. efficacy and safety in a retrospective study of 63 patients. J. Eur. Acad. Dermatol. Venereol. 2017. 31(5). 837-842. doi: 10.1111/jdv.14066.
- Sarıcaoğlu H., Yazici S., Zorlu Özge., Bülbül Başkan E., Aydoğan K. Cyclosporine-A for severe childhood atopic dermatitis: clinical experience on efficacy and safety profile. Turk. J. Med. Sci. 2018. 48(5). 933-938. doi: 10.3906/sag-1711-7.
- Deo M., Yung A., Hill S., Rademaker M. Methotrexate for treatment of atopic dermatitis in children and adolescents. Int. J. Dermatol. 2014. 53(8). 1037-1041. doi: 10.1111/ijd.12314.
- Dvorakova V., O’Regan G.M., Irvine A.D. Methotrexate for severe childhood atopic dermatitis: clinical experience in a tertiary center. Pediatr. Dermatol. 2017. 34(5). 528-534.doi: 10.1111/pde.13209.
- Taieb Y., Baum S., Ben Amitai D., Barzilai A., Greenberger S. The use of methotrexate for treating childhood atopic dermatitis: a multicenter retrospective study. J. Dermatolog. Treat. 2019. 30(3). 240-244. doi: 10.1080/09546634.2018.1508816.
- Noguera-Morel L., Knöpfel N., Torrelo A., Hernández-Martín A. A retrospective study of systemic treatment of severe atopic dermatitis with azathioprine: effectiveness and tolerance in 11 pediatric patients. Actas Dermo-Sifiliográficas. 2019. 110(3). 227-231. doi: 10.1016/j.adengl.2018.06.027.
- Fuggle N.R., Bragoli W., Mahto A., Glover M., Martinez A.E., Kinsler V.A. The adverse effect profile of oral azathioprine in pediatric atopic dermatitis, and recommendations for monitoring. J. Am. Acad. Dermatol. 2015. 72(1). 108-114.doi: 10.1016/j.jaad.2014.08.048.
- Wollenberg A., Oranje A., Deleuran M. et al. ETFAD/EADV eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J. Eur. Acad. Dermatol. Venereol. 2016. 30(5). 729-747. doi: 10.1111/jdv.13599.
- Drucker AM., Eyerich K., de Bruin-Weller M.S. et al. Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br. J. Dermatol. 2018. 178(3). 768-775. doi: 10.1111/bjd.15928.
- Simpson E.L., Paller A.S., Siegried E.C. et al. Dupilumab efficacy and safety in adolescents with moderate-to-severe atopic dermatitis: results from a multicenter, randomized, placebo-controlled, double-blind, parallel-group, phase 3 study. 27th EADV Congress, September 12–16, 2018. Paris, France: Abstract № 4640; 2018.
- Treister A.D., Lio P.A. Long-term off-label dupilumab in pediatric atopic dermatitis: a case series. Pediatr. Dermatol. 2019. 36(1). 85-88. doi: 10.1111/pde.13697.
- Siegfried EC., Igelman S., Jaworsk J.C. et al. Use of dupilimab in pediatric atopic dermatitis: access, dosing, and implications for managing severe atopic dermatitis. Pediatr. Dermatol. 2019. 36(1). 172-176. doi: 10.1111/pde.13707.
- Gupta R.S., Warren C.M., Smith B.M. et al. Prevalence and severity of food allergies among US adults. JAMA Netw. Open. 2019. 2(1).
- Jackson K.D., Howie L.D., Akinbami L.J. Trends in allergic conditions among children: United States, 1997–2011. NCHS data brief, no 121. Hyattsville, MD: National Center for Health Statistics; 2013. 1-8.
- Nwaru B.I., Hickstein L., Panesar S.S., Roberts G., Muraro A., Sheikh A. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014. 69. 992-1007.
- Osborne N.J., Koplin J.J., Martin P.E. et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J. Allergy Clin. Immunol. 2011. 127. 668-676.
- Rinaldi M., Harnack L., Oberg C., Schreiner P., St Sauver J., Travis L.L. Peanut allergy diagnoses among children residing in Olmsted County, Minnesota. J. Allergy Clin. Immunol. 2012. 130. 945-950.
- Sicherer S.H., Munoz-Furlong A., Godbold J.H., Sampson H.A. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J. Allergy Clin. Immunol. 2010. 125. 1322-1326.
- Soller L., Ben-Shoshan M., Harrington D.W. et al. Overall pre–valence of self-reported food allergy in Canada. J. Allergy Clin. Immunol. 2012. 130. 986-988.
- Шадрін О.Г., Няньковський С.Л., Добрянський Д.О. та ін. Особливості діагностики та підходи до лікувально-профілактичного харчування дітей раннього віку з алергією до білка коров’ячого молока: метод. рекоменд. К.: Люди в білому, 2014. 28 с.
- Sicherer S.H., Sampson H.A. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014. 133. 291-307.
- Fleischer D.M., Greenhawt M., Sussman G. et al. Effect of epicutaneous immunotherapy vs placebo on reaction to peanut protein ingestion among children with peanut allergy: The PEPITES randomized clinical trial. JAMA. 2019. 321(10). 946.
- Vickery B.P., Vereda A., Casale T.B. et al. AR101 oral immunotherapy for peanut allergy. N. Engl. J. Med. 2018. 379. 1991-2001.
- Andorf S., Purington N., Kumar D. et al. A phase 2 randomized controlled multisite study using omalizumab-facilitated rapid desensitization to test continued vs discontinued dosing in multifood allergic individuals. EClinicalMedicine. 2019. 7. 27-38.
- Sampson H.A., Leung D.Y., Burks A.W. et al. A phase II, randomized, double-blind, parallel-group, placebo-controlled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J. Allergy Clin. Immunol. 2011. 127(5). 1309-1310.
- Nowak-Wegrzyn A., Fiocchi A. Is oral immunotherapy the cure for food allergies? Curr. Opin. Allergy Clin. Immunol. 2010. 10. 214-219.
- Rolinck-Werninghaus C., Staden U., Mehl A., Hamelmann E., Beyer K., Niggemann B. Specific oral tolerance induction with food in children: transient or persistent effect on food allergy? Allergy. 2005. 60. 1320-1322.
- Hussey Freeland D.M., Fan-Minogue H., Spergel J.M., Chatila T.A., Nadeau K.C. Advances in food allergy oral immunotherapy: toward tolerance. Curr. Opin. Immunol. 2016. 42. 119-123.
- Sampath V., Sindher S.B., Pinzon A.M.A., Nadeau K.C. Can food allergy be cured? What are the future prospects? Allergy. 2020. Vol. 75. P. 1316-1326.
- Wood R.A., Sicherer S.H., Burks A.W. et al. A phase 1 study of heat/phenol-killed, E. coli-encapsulated, recombinant modified peanut proteins Ara h 1, Ara h 2, and Ara h 3 (EMP-123) for the treatment of peanut allergy. Allergy. 2013. 68. 803-808.
- Dhar S., Larché M. PVX108 peptide immunotherapy significantly reduces markers of peanut-induced anaphylaxis in a dose-dependent manner. J Allergy Clin. Immunol. 2019. 143(2). AB426.

/33.jpg)