Международный эндокринологический журнал Том 17, №7, 2021
Вернуться к номеру
Цитокины в крови больных сахарным диабетом 2-го типа в зависимости от величины избыточной массы тела/ожирения (анализ литературы и собственных исследований)
Авторы: Зак К.П., Попова В.В., Орленко В.Л., Фурманова О.В., Тронько Н.Д.
ГУ «Институт эндокринологии и обмена веществ им. В.П. Комиссаренко НАМН Украины», г. Киев, Украина
Рубрики: Эндокринология
Разделы: Справочник специалиста
Версия для печати
У роботі наведено аналіз сучасних даних літератури та результати власних досліджень щодо стану цитокінової мережі: про- та антизапальних цитокінів (інтерлейкіни (ІЛ) 1α, ІЛ-1β, ІЛ-4, ІЛ-6, ІЛ-10, ІЛ-17 та фактор некрозу пухлини альфа — ФНОα), α- та β-хемокінів, у тому числі ІЛ-8 та ІЛ-16, а також адипокінів (лептин та адипонектин) у периферичній крові хворих на цукровий діабет 2-го типу (ЦД-2) з нормальною та підвищеною масою тіла/ожирінням. Показано, що для хворих на ЦД-2 характерний підвищений вміст у периферичній крові прозапальних цитокінів (ІЛ-1, ІЛ-6, ІЛ-17, ФНПα) та α- і β-хемокінів, у тому числі ІЛ-8 та ІЛ-16, а також лептину при зниженні вмісту адипонектину. У худих хворих (індекс маси тіла (ІМТ) < 25,5 кг/м2) порівняно з худими нормоглікемічними особами контрольної групи (ІМТ < 25,5 кг/м2) відзначається невелике, але вірогідне підвищення ІЛ-1β, ІЛ-6, ІЛ-17, ФНПα та лептину, що в міру збільшення ІМТ значно зростає при вираженому ожирінні (ІМТ > 30,0 кг/м2), особливо у гладких жінок (ІМТ > 35,0 кг/м2). Аналогічно збільшення вмісту прозапальних цитокінів спостерігається і в нормоглікемічних людей, але не таке значне, як при ЦД-2. Менш чіткі дані були отримані при визначенні протизапальних цитокінів ІЛ-4 та ІЛ-10, що пояснюється значним поліморфізмом їх генів, як захисною, так і компенсаторною відповіддю на підйом рівня прозапальних цитокінів. У хворих на ЦД-2, особливо з ожирінням, спостерігається підвищений рівень лептину зі зниженням рівня адипонектину. Тяжкість перебігу та відсоток смертності тісно асоційовані з величиною ІМТ хворих. Боротьба зі збільшенням частоти захворюваності на ЦД-2 повинна бути в першу чергу спрямована на профілактику ожиріння, а при вже розвиненому захворюванні на ЦД-2 — на зниження супутнього ожиріння. Аналіз даних наводить також на думку, що різке підвищення вмісту прозапальних цитокінів («цитокіновий шторм»), що спостерігається у хворих на ЦД-2 та ожиріння, інфікованих COVID-19, є наслідком підсумовування та потенціювання вже наявного раніше запального процесу.
В работе приведены анализ современных данных литературы и результаты собственных исследований, касающихся состояния цитокиновой сети: про- и антивоспалительных цитокинов (интерлейкин (ИЛ) 1α, ИЛ-1β, ИЛ-4, ИЛ-6, ИЛ-10, ИЛ-17 и фактор некроза опухоли альфа — ФНОα), α- и β-хемокинов, в том числе ИЛ-8 и ИЛ-16, а также адипокинов (лептин и адипонектин) в периферической крови больных сахарным диабетом 2-го типа (СД-2) с нормальной и повышенной массой тела/ожирением. Показано, что для больных СД-2 характерно повышенное содержание в периферической крови провоспалительных цитокинов (ИЛ-1, ИЛ-6, ИЛ-17, ФНОα) и α- и β-хемокинов, в том числе ИЛ-8 и ИЛ-16, а также лептина при снижении содержания адипонектина. У худых больных (индекс массы тела (ИМТ) < 25,5 кг/м2) по сравнению с худыми нормогликемическими лицами контрольной группы (ИМТ < 25,5 кг/м2) отмечается небольшое, но достоверное повышение ИЛ-1β, ИЛ-6, ИЛ-17, ФНОα и лептина, которое по мере увеличения ИМТ значительно возрастает при выраженном ожирении (ИМТ > 30,0 кг/м2), особенно у тучных женщин (ИМТ > 35,0 кг/м2). Аналогично увеличение содержания провоспалительных цитокинов наблюдается и у нормогликемических людей, но не такое значительное, как при СД-2. Менее четкие данные были получены при определении антивоспалительных цитокинов ИЛ-4 и ИЛ-10, что объясняется значительным полиморфизмом их генов, как защитным, так и компенсаторным ответом на подъем уровня провоспалительных цитокинов. У больных СД-2, особенно с ожирением, наблюдается повышенный уровень лептина со снижением уровня адипонектина. Тяжесть течения и процент смертности тесно ассоциированы с величиной ИМТ больных. Борьба с увеличением частоты заболеваемости СД-2 должна быть в первую очередь направлена на профилактику ожирения, а при уже развившемся заболевании СД-2 — на снижение сопутствующего ожирения. Анализ приведенных данных наводит также на мысль, что резкое повышение содержания провоспалительных цитокинов («цитокиновый шторм»), наблюдаемое у больных СД-2 и ожирением, инфицированных COVID-19, является следствием суммирования и потенцирования уже имеющегося ранее воспалительного процесса.
The paper analyzes the current literature data and the results of our own researches concerning the state of the cytokine network: pro- and anti-inflammatory cytokines (interleukin (IL) 1α, IL-1β, IL-4, IL-6, IL-10, IL-17 and tumor necrosis factor (TNF) α), α- and β-chemokines, including IL-8 and IL-16, as well as adipokines (leptin and adiponectin) in the peripheral blood of patients with type 2 diabetes (T2D) with normal and increased body weight/obesity. It has been shown that patients with T2D are characterized by an increased content of proinflammatory cytokines (IL-1, IL-6, IL-17, TNFα), α- and β-chemokines in the peripheral blood, including IL-8 and IL-16, as well as leptin with a decrease in adiponectin content. In lean patients (with body mass index (BMI) < 25.5 kg/m2) compared to lean normoglycemic individuals from the control group (BMI < 25.5 kg/m2), there is a small but significant increase in IL-1β, IL-6, IL-17, TNFα and leptin, which, as BMI increases, significantly increases in severe obesity (BMI > 30.0 kg/m2), especially in obese women (BMI > 35.0 kg/m2). Similarly, an increase in proinflammatory cytokines is observed in normoglycemic people, but not as significant as in T2D. Less clear data were obtained when during determination of the anti-inflammatory cytokines IL-4 and IL-10, which is explained by a significant polymorphism of their genes, and both protective and compensatory effects on pro-inflammatory cytokine rise. In T2D patients, especially those with obesity, there is an increase in the leptin level and a decrease in the adiponectin content. The severity of the course and the percentage of mortality are closely associated with the BMI of patients. The effectiveness of the fight against an increase in the incidence of T2D should be primarily aimed at preventing obesity, and in case of already developed T2D — at reducing concomitant obesity. The analysis of the data presented also suggests that a sharp increase in the content of pro-inflammatory cytokines (so called cytokine storm) observed in patients with T2D and obesity infected with COVID-19, is a consequence of the summation and potentiation of already existing inflammatory process.
цукровий діабет 2-го типу; імунітет; цитокіни; COVID-19
сахарный диабет 2-го типа; иммунитет; цитокины; COVID-19
type 2 diabetes mellitus; immunity; cytokines; COVID-19
Введение
Цитокины у больных СД-2 с нормальной массой тела
Цитокины у нормогликемических лиц с ожирением
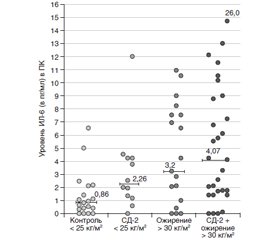
Цитокины у больных СД-2 с повышенной массой тела/ожирением
Заключение
- Paul U.Ye. Immunologiia. Tom 1 [Immunology. Vol. 1]. Moskva: Mir, 1987–1988. 472 p. (in Russian).
- Vozianov A.F., Butenko A.K., Zak K.P. Cytokines. Biological and antitumor properties. Kiev: Naukova Dumka, 1998. 320 p. (in Russian).
- Zak K.P., Tron’ko N.D., Popova V.V., Butenko A.K. Diabetes mellitus. Immunity. Cytokines. Kiev: Knyga-pljus, 2015. 485 p. (in Russian).
- Tron'ko M.D., Zak K.P. Current advances in clinical pathophysiology in the study of the pathogenesis of type 1 and type 2 diabetes mellitus in humans. Mìžnarodnij endokrinologìčnij žurnal. 2019. 15(6). 422-34. doi: 10.22141/2224-0721.15.6.2019.185403 (in Russian).
- O'Shea J.J., Paul W.E. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010, Feb. 26. 327(5969). 1098-102. doi: 10.1126/science.1178334.
- Sabat I.E., Lindsey A.P., Membere A., Anderson A., Ahmad A., King E., Bolunmez B. Invisible disabilities: unique strategies for workplace allies. Industrial and Organizational Psychology. 2014, Jun. 7(2). 259-65. https:// doi.org/10.1111/iops.12145.
- Zak K.P., Gruzov M.I., Khomenko B.M., Shlyakhovenko V.S. Ultrastructure of large granulo-containing lymphocytes of human blood. Doklady AN URSR. 1983. 4. 73-7.
- Hedjazifar S., Khatib Shahidi R., Hammarstedt A., Bonnet L., Church C., Boucher J. et al. The novel adipokine gremlin 1 antagonizes insulin action and is increased in type 2 diabetes and NAFLD/NASH. Diabetes. 2020, Mar. 69(3). 331-41. doi: 10.2337/db19-0701.
- Kondratska I., Melnichenko S., Mankovsky B., Zak K. Serum interleukin-16 levels in patients with metabolic syndrome and diabetes mellitus. J. Clin. Lipidology. 2008. 2(5). 104.
- Mello V.D.F., Kolehmainen M., Schwab U., Mager U., Laaksonen D.E., Pulkkinen L. et al. Weight reduction decreases TNF-α and increases IL-6 gene expression in lymphocytes in subjects with metabolic syndrome — the GENOBIN study. Diabetologia. 2006. 49(Suppl. 1). 466. http://hdl.handle. net/10183/11324.
- Zhang Z., Yuan W., Sun L., Szeto F.L., Wong K.E., Li X. et al. 1,25-Dihydroxyvitamin D3 targeting of NF-kappaB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int. 2007, Jul. 72(2). 193-201. doi: 10.1038/sj.ki.5002296.
- Badr E., Assar M., Elshayeb E.I., Fath El-Bab S., El-Kousy S. A preliminary study of the relation between IL-4 and hypertension in type II diabetes mellitus. Mol. Biol. Rep. 2018, Dec. 45(6). 1967-1972. doi: 10.1007/s11033-018-4349-7.
- Rodrigues K.F., Pietrani N.T., Bosco A.A., Campos F.M.F., Sandrim V.C., Gomes K.B. IL-6, TNF-α, and IL-10 levels/polymorphisms and their association with type 2 diabetes mellitus and obesity in Brazilian individuals. Arch. Endocrinol. Metab. 2017, Sept-Oct. 61(5). 438-46. doi: 10.1590/2359-3997000000254.
- Williams A., Greene N., Kimbro K. Increased circulating cytokine levels in African American women with obesity and elevated HbA1c. Cytokine. 2020 Apr. 128. 154989. doi: 10.1016/j.cyto.2020.154989.
- International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels, Belgium: 2019. Available at: https. //www.diabetesatlas.org.
- Bommer C., Sagalova V., Heesemann E., Manne-Goehler J., Atun R., Bärnighausen T. et al. Global economic burden of diabetes in adults: Projections from 2015 to 2030. Diabetes Care. 2018, May. 41(5). 963-70. doi: 10.2337/dc17-1962.
- Donath M.Y. Multiple benefits of targeting inflammation in the treatment of type 2 diabetes. Diabetologia. 2016, Apr. 59(4). 679-82. doi: 10.1007/s00125-016-3873-z.
- Donath M.Y., Shoelson S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, Feb. 11(2). 98-107. doi: 10.1038/nri2925.
- Netea M.G., Balkwill F., Chonchol M., Cominelli F., Donath M.Y., Giamarellos-Bourboulis E.J. et al. A guiding map for inflammation. Nat. Immunol. 2017, Jul. 19. 18(8). 826-31. doi: 10.1038/ni.3790. Erratum in: Nat. Immunol. 2021, Feb. 22(2). 254.
- Duncan B.B., Schmidt M.I., Pankow J.S., Ballantyne C.M., Couper D., Vigo A. et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003, Jul. 52(7). 1799-805. doi: 10.2337/diabetes.52.7.1799.
- Hu F.B., Meigs J.B., Li T.Y., Rifai N., Manson J.E. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004, Mar. 53(3). 693-700. doi: 10.2337/diabetes.53.3.693.
- Furmanova O.V., Zak K.P., Popova V.V., Tron’ko N.D. Blood leukocyte composition and neutrophils to lymphocyte ratio in patients with newly diagnosed type 2 diabetes mellitus depending on the degree of overweight/obesity. Mìžnarodnij endokrinologìčnij žurnal. 2020. 16(7). 526-33. doi: 10.22141/2224-0721.16.7.2020.219006 (in Russian).
- Alexandraki K., Piperi C., Kalofoutis C., Singh J., Alaveras A., Kalofoutis A. Inflammatory process in type 2 diabetes: The role of cytokines. Ann. N. Y. Acad. Sci. 2006, Nov. 1084. 89-117. doi: 10.1196/annals.1372.039.
- Dinarello C.A., Donath M.Y., Mandrup-Poulsen T. Role of IL-1beta in type diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2010. Aug. 17(4). 314-21. doi: 10.1097/MED.0b013e32833bf6dc.
- Mirza S., Hossain M., Mathews C., Martinez P., Pino P., Gay J.L. et al. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cytokine. 2012, Jan. 57(1). 136-42. doi: 10.1016/j.cyto.2011.09.029.
- Donath M.Y., Dalmas É., Sauter N.S., Böni-Schnetzler M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab. 2013, Jun. 4. 17(6). 860-72. doi: 10.1016/j.cmet.2013.05.001.
- Guarino D., Antonioli L., Fornai M., Pellegrini C., Blandizzi C., Anselmino M. et al. Diabetes, obesity and inflammation: Persistence of elevated IL-1b after bariatric surgery. Diabetologia. 2017. 60 (Suppl. 1). 1-97.
- Herder C., Zierer A., Koenig W., Roden M., Meisinger C., Thorand B. Transforming growth factor-beta1 and incident type 2 diabetes: results from the MONICA/KORA case-cohort study, 1984–2002. Diabetes Care. 2009, Oct. 32(10). 1921-3. doi: 10.2337/dc09-0476.
- Larsen C.M., Faulenbach M., Vaag A. et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007, Apr. 12. 356(15). 1517-26. doi: 10.1056/NEJMoa065213.
- Mandrup-Poulsen T. Interleukin-1 antagonism: a study companion for immune tolerance induction in type 1 diabetes? Diabetes. 2014, Jun. 63(6). 1833-5. doi: 10.2337/db14-0371.
- Pham M.N., Hawa M.I., Pfleger C., Roden M., Schernthaner G., Pozzilli P. et al. Pro- and anti-inflammatory cytokines in latent autoimmune diabetes in adults, type 1 and type 2 diabetes patients: Action LADA 4. Diabetologia. 2011, Jul. 54(7). 1630-8. doi: 10.1007/s00125-011-2088-6. Epub 2011 Feb 24. Erratum in: Diabetologia. 2012 Feb. 55(2). 534. Scherbaum W. [corrected to Scherbaum W.A.].
- Spranger J., Kroke A., Möhlig M., Hoffmann K., Bergmann M.M., Ristow M. et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003, Mar. 52(3). 812-7. doi: 10.2337/diabetes.52.3.812.
- Wannamethee S.G., Papacosta O., Lawlor D.A., Whincup P.H., Lowe G.D., Ebrahim S., Sattar N. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women’s Heart Health Study. Diabetologia. 2012, Jan. 55(1). 80-7. doi: 10.1007/s00125-011-2284-4.
- Jafar N., Edriss H., Nugent K. The effect of short-term hyperglycemia on the innate immune system. Am. J. Med. Sci. 2016, Feb. 351(2). 201-11. doi: 10.1016/j.amjms.2015.11.011.
- Dandona P., Aljada A., Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004, Jan. 25(1). 4-7. doi: 10.1016/j.it.2003.10.013.
- Banerjee M., Saxena M. Interleukin-1 (IL-1) family of cytokines: role in type 2 diabetes. Clin. Chim. Acta. 2012, Aug. 16. 413(15-16). 1163-70. doi: 10.1016/ j.cca.2012.03.021.
- Dror E., Dalmas E., Meier D.T., Wueest S., Thévenet J., Thienel C. et al. Postprandial macrophage-derived IL-1β stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat. Immunol. 2017, Mar. 18(3). 283-92. doi: 10.1038/ni.3659.
- Herder C., Brunner E., Tabak A. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist (IL-1Ra) precede, but do not prevent, the onset of type 2 diabetes (The Whitehall II Study). In: Minutes of The 43rd General Assembly of The European Association for The Study of Diabetes. Diabetologia. 2008. 51(Suppl. 1). 313. doi: 10.1007/s00125-008-1117-6.
- Carstensen M., Herder C., Kivimäki M., Jokela M., Roden M., Shipley M.J. et al. Accelerated increase in serum interleukin-1 receptor antagonist starts 6 years before diagnosis of type 2 diabetes: Whitehall II prospective cohort study. Diabetes. 2010, May. 59(5). 1222-7. doi: 10.2337/db09-1199.
- Herder C., Baumert J., Thorand B., Martin S., Löwel H., Kolb H., Koenig W. Elevated systemic chemokine concentrations precede the incidence of coronary heart disease and type 2 diabetes: results from the MONICA/KORA Augsburg study, 1984–2002. Arterioscler. Thromb. Vasc. Biol. 2006, Sep. 26(9). 2147-52. doi: 10.1161/01.ATV.0000235691.84430.86.
- Urwyler S.A., Schuetz P., Ebrahimi F., Donath M.Y., Christ-Crain M. Interleukin-1 antagonism decreases cortisol levels in obese individuals. J. Clin. Endocrinol. Metab. 2017, May 1. 102(5). 1712-8. doi: 10.1210/jc.2016-3931.
- Cartier A., Lemieux I., Alméras N., Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. Diabetologia. 2007. 50(Suppl. 1). 270.
- Goyal R., Faizy A.F., Siddiqui S.S., Singhai M. Evaluation of TNF-α and IL-6 levels in obese and non-obese diabetics: pre- and postinsulin effects. N. Am. J. Med. Sci. 2012, Apr. 4(4). 180-4. doi: 10.4103/1947-2714.94944.
- Hansen D., Dendale P., Beelen M., Jonkers R.A., Mullens A., Corluy L. et al. Plasma adipokine and inflammatory marker concentrations are altered in obese, as opposed to non-obese, type 2 diabetes patients. Eur. J. Appl. Physiol. 2010, Jun. 109(3). 397-404. doi: 10.1007/s00421-010-1362-5.
- Herder C., Zierer A., Koenig W. Elevated serum levels of transforming growth factor-beta1 (TGFbeta-1) precede the development of type 2 diabetes: MONICA/KORA Augsburg case-cohort study, 1984–2002. Diabetologia. 2009. 52 (Suppl. 1). A-837.
- Thorand B., Kolb H., Baumert J., Koenig W., Chambless L., Meisinger C. et al. Elevated levels of interleukin-18 predict the development of type 2 diabetes: results from the MONICA/KORA Augsburg Study, 1984–2002. Diabetes. 2005, Oct. 54(10). 2932-8. doi: 10.2337/diabetes.54.10.2932.
- Zak K.P., Mankovsky B.N., Kondratska I.N. et al. Immunity in patients with type 2 diabetes mellitus with concomitant metabolic syndrome/obesity. Communication 1. Composition of blood leukocytes, immunophenotype of lymphocytes, and ultrastructure of neutrophils. Endokrynologia. 2013. 18(1). 27-36 (in Russian).
- Ellingsgaard H., Hauselmann I., Schuler B., Habib A.M., Baggio L.L., Meier D.T. et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011, Oct. 30. 17(11). 1481-9. doi: 10.1038/nm.2513.
- Wueest S., Laesser C.I., Böni-Schnetzler M., Item F., Lucchini F.C., Borsigova M. et al. IL-6-type cytokine signaling in adipocytes induces intestinal GLP-1 secretion. Diabetes. 2018, Jan. 67(1). 36-45. doi: 10.2337/db17-0637.
- Orlenko V.L., Zak K.P. Treatment with glucagon-like peptide-1 analogues: a breakthrough in diabetes mellitus type 2 therapy. Mìžnarodnij endokrinologìčnij žurnal. 2014. (60). 112-7. doi: 10.22141/2224-0721.4.60.2014.76690 (in Russian).
- Wang X., Bao W., Liu J., Ouyang Y.Y., Wang D., Rong S. et al. Inflammatory markers and risk of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care. 2013, Jan. 36(1). 166-175. doi: 10.2337/dc12-0702.
- Daniele G., Guardado Mendoza R., Winnier D., Fiorentino T.V., Pengou Z., Cornell J. et al. The inflammatory status score including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and adiponectin underlies whole-body insulin resistance and hyperglycemia in type 2 diabetes mellitus. Acta Diabetol. 2014, Feb. 51(1). 123-31. doi: 10.1007/s00592-013-0543-1.
- Hang H., Yuan S., Yang Q., Yuan D., Liu Q. Multiplex bead array assay of plasma cytokines in type 2 diabetes mellitus with diabetic retinopathy. Mol. Vis. 2014, Aug 4. 20. 1137-45. PMID: 25253986; PMCID: PMC4124102.
- Kopecky J., Krusinova E., Wohl P. Effect of 24-hour hypertriglyceridaemia on tumor necrosis factor alpha and resistin in type 2 diabetes and healthy subjects. Diabetologia. 2008. 51(Suppl. 1). 322.
- Lindmark S., Burén J., Eriksson J.W. Insulin resistance, endocrine function and adipokines in type 2 diabetes patients at different glycaemic levels: potential impact for glucotoxicity in vivo. Clin. Endocrinol. (Oxf.). 2006, Sep. 65(3). 301-9. doi: 10.1111/j.1365-2265.2006.02593.x.
- Mishima Y., Kuyama A., Tada A., Takahashi K., Ishioka T., Kibata M. Relationship between serum tumor necrosis factor-alpha and insulin resistance in obese men with Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2001, May. 52(2). 119-23. doi: 10.1016/s0168-8227(00)00247-3.
- Su S.C., Pei D., Hsieh C.H., Hsiao F.C., Wu C.Z., Hung Y.J. Circulating pro-inflammatory cytokines and adiponectin in young men with type 2 diabetes. Acta Diabetol. 2011, Jun. 48(2). 113-9. doi: 10.1007/s00592-009-0171-y.
- Zak K.P., Mankovsky B.N., Melnichenko S.V. et al. Immunity in patients with type 2 diabetes mellitus in complex with concomitant metabolic syndrome/obesity. Communication 2. Role of adipocytokines (interleukin-6, tumor necrosis factor alpha, leptin and adiponectin). Endokrynologia. 2013. 18(2). 26-32 (in Russian).
- Zak K.P., Furmanova O.V. Immune and anti-inflammatory factors in the mechanism of therapeutic effect of metformin in type 2 diabetes mellitus (analytical review including the results of own researches). Mìžnarodnij endokrinologìčnij žurnal. 2018. 14(2). 173-81. doi: 10.22141/2224-0721.14.2.2018.130564 (in Ukrainian).
- Zhao Z., Tang X., Song K., Li X., Zhang Y. Association of -308G/A and -238G/A polymorphisms of TNF-α and osteosarcoma risk. Int. J. Clin. Exp. Pathol. 2015, Apr. 1. 8(4). 4177-81.
- Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., Weaver C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, Nov. 6(11). 1123-32. doi: 10.1038/ni1254.
- Park H., Li Z., Yang X.O., Chang S.H., Nurieva R., Wang Y.H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, Nov. 6(11). 1133-41. doi: 10.1038/ni1261.
- Zak K.P., Popova V.V. The role of IL-17 in the pathogenesis of type 1 and type 2 diabetes mellitus in humans. Mìžnarodnij endokrinologìčnij žurnal. 2018. 14(5). 514-21. doi: 10.22141/2224-0721.14.5.2018.142690 (in Russian).
- Fatima N., Faisal S.M., Zubair S., Siddiqui S.S., Moin S., Owais M. Emerging role of nterleukins IL-23/IL-17 axis and biochemical markers in the pathogenesis of type 2 diabetes: association with age and gender in human subjects. Int. J. Biol. Macromol. 2017, Dec. 105(Pt 1). 1279-88. doi: 10.1016/j.ijbiomac.2017.07.155.
- Fores J.P., Crisostomo L.G., Orii N.M., Santos A.S., Fukui R.T., Matioli S.R. et al. Th17 pathway in recent-onset autoimmune diabetes. Cell. Immunol. 2018, Feb. 324. 8-13. doi: 10.1016/j.cellimm.2017.11.005.
- Zhang C., Xiao C., Wang P., Xu W., Zhang A., Li Q., Xu X. The alteration of Th1/Th2/Th17/Treg paradigm in patients with type 2 diabetes mellitus: Relationship with diabetic nephropathy. Hum. Immunol. 2014, Apr. 75(4). 289-96. doi: 10.1016/j.humimm.2014.02.007.
- Chen C., Shao Y., Wu X., Huang C., Lu W. Elevated interleukin-17 levels in patients with newly diagnosed type 2 diabetes mellitus. Biochem. Physiol. 2016. 5(206). 2-10. doi: 10.4172/2168-9652.1000206.
- Fabbrini E., Cella M., McCartney S.A., Fuchs A., Abumrad N.A., Pietka T.A. et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013, Aug. 145(2). 366-74.e1-3. doi: 10.1053/j.gastro.2013.04.010.
- Ohashi K., Ouchi N., Matsuzawa Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie. 2012, Oct. 94(10). 2137-42. doi: 10.1016/j.biochi.2012.06.008.
- Roohi A., Tabrizi M., Abbasi F., Ataie-Jafari A., Nikbin B., Larijani B. et al. Serum IL-17, IL-23, and TGF-β levels in type 1 and type 2 diabetic patients and age-matched healthy controls. Biomed. Res. Int. 2014. 718946. doi: 10.1155/2014/718946.
- Zak K.P., Furmanova O.V., Popova V.V., Sayenko Ya.A. The content of pro-inflammatory cytokines IL-1β, IL-6, IL-17A and TNFα in the blood of patients with type 2 diabetes after therapy with metformin. Ukr. Biochem. J. 2020. 92(6). 105-12. https://doi.org/10.15407/ubj92.06.105.
- Abdel-Moneim A., Bakery H.H., Allam G. The potential pathogenic role of IL-17/Th17 cells in both type 1 and type 2 diabetes mellitus. Biomed. Pharmacother. 2018, May. 101. 287-92. doi: 10.1016/j.biopha.2018.02.103.
- Ho K.T., Shiau M.Y., Chang Y.H., Chen C.M., Yang S.C., Huang C.N. Association of interleukin-4 promoter polymorphisms in Taiwanese patients with type 2 diabetes mellitus. Metabolism. 2010, Dec. 59(12). 1717-22. doi: 10.1016/j.metabol.2010.04.010.
- Silva-Filho J.L., Caruso-Neves C., Pinheiro A.A.S. IL-4: an important cytokine in determining the fate of T cells. Biophys. Rev. 2014, Mar. 6(1). 111-8. doi: 10.1007/s12551-013-0133-z.
- Sartangello C., Marchetti P., Marselli L. Suppressors of cytokine signaling (SOCS) in cytokine-induced human islet cell damage. Diabetologia. 2001. 44(Suppl. 1). A-41.
- Te Velde A.A., Huijbens R.J., Heije K., de Vries J.E., Figdor C.G. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood. 1990, Oct. 1. 76(7). 1392-7. PMID: 2119829.
- Cheung D.L., Hart P.H., Vitti G.F., Whitty G.A., Hamilton J.A. Contrasting effects of interferon-gamma and interleukin-4 on the interleukin-6 activity of stimulated human monocytes. Immunology. 1990, Sep. 71(1). 70-5. PMID: 2120129. PMCID; PMC1384223.
- Binisor I.D., Moldovan R., Moldovan I., Andrei A.M., Ba–nita M.I. Abdominal obesity and type 2 diabetes mellitus are associated with higher seric levels of IL 4 in adults. Curr. Health Sci. J. 2016, Jul-Sep. 42(3). 231-7. doi: 10.12865/CHSJ.42.03.03.
- Shiau M.Y., Chuang P.H., Yang C.P., Hsiao C.W., Chang S.W. et al. Mechanism of interleukin-4 reducing lipid deposit by regulating hormone-sensitive lipase. Sci. Rep. 2019, Aug. 19. 9(1). 11974. doi: 10.1038/s41598-019-47908-9.
- Alsaid A., El-Missiry M., Hatata El-S., Tarabay M., Settin A. Association of IL-4-590 C > T and IL-13-1112 C > T gene polymorphisms with the susceptibility to type 2 diabetes mellitus. Dis. Markers. 2013. 35(4). 243-7. doi: 10.1155/2013/107470.
- Blüher M., Fasshauer M., Tönjes A., Kratzsch J., Schön M.R., Paschke R. Association of interleukin-6, C-reactive protein, interleukin-10 and adiponectin plasma concentrations with measures of obesity, insulin sensitivity and glucose metabolism. Exp. Clin. Endocrinol. Diabetes. 2005, Oct. 113(9). 534-7. doi: 10.1055/s-2005-872851.
- Esposito K., Pontillo A., Giugliano F., Giugliano G., Marfella R., Nicoletti G., Giugliano D. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J. Clin. Endocrinol. Metab. 2003, Mar. 88(3). 1055-8. doi: 10.1210/jc.2002-021437.
- Van Exel E., Gussekloo J., de Craen A.J., Frölich M., Bootsma-Van Der Wiel A., Westendorp R.G. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes: the Leiden 85-Plus Study. Diabetes. 2002, Apr. 51(4). 1088-92. doi: 10.2337/diabetes.51.4.1088.
- Straczkowski M., Kowalska I., Nikolajuk A., Krukowska A., Gorska M. Plasma interleukin-10 concentration is positively related to insulin sensitivity in young healthy individuals. Diabetes Care. 2005, Aug. 28(8). 2036-7. doi: 10.2337/diacare.28.8.2036.
- Kim Y.H., Pyo S. Interleukin-10 suppresses adipogenesis via Wnt5a signaling pathway in 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2019, Feb. 19. 509(4). 877-85. doi: 10.1016/j.bbrc.2019.01.033.
- Canecki-Varžić S., Prpić-Križevac I., Mihaljević S., Bilić-Ćurčić I., Alkhamis T., Wagner J. et al. Association between interleukin-10 gene (-1082g/A) polymorphism and type 2 diabetes, diabetes-related traits, and microvascular complications in the Croatian population. Acta Clin. Croat. 2018, Mar. 57(1). 71-81. doi: 10.20471/acc.2018.57.01.08.
- Zhang Z., Yuan W., Sun L., Szeto F.L., Wong K.E., Li X. et al. 1,25-Dihydroxyvitamin D3 targeting of NF-kappaB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int. 2007, Jul. 72(2). 193-201. doi: 10.1038/sj.ki.5002296.
- Coll B., Alonso-Villaverde C., Joven J. Monocyte chemoattractant protein-1 and atherosclerosis: is there room for an additional biomarker? Clin. Chim. Acta. 2007, Aug. 383(1-2). 21-9. doi: 10.1016/j.cca.2007.04.019.
- Chow F.Y., Nikolic-Paterson D.J., Ozols E., Atkins R.C., Rollin B.J., Tesch G.H. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006, Jan. 69(1). 73-80. doi: 10.1038/sj.ki.5000014.
- Zorena K., Mysliwska J., Lipowski P. Chemokines in aqueous humor and serum in patients with cataract and proliferative diabetic retinopathy: a preliminary study. J. Diabetes. 2009. 1(Suppl. 1). A-165.
- Müller S., Martin S., Koenig W., Hanifi-Moghaddam P., Rathmann W., Haastert B. et al. Impaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute-phase proteins but not TNF-alpha or its receptors. Diabetologia. 2002, Jun. 45(6). 805-12. doi: 10.1007/s00125-002-0829-2.
- Zozuliñska D., Majchrzak A., Sobieska M., Wiktorowicz K., Wierusz-Wysocka B. Serum interleukin-8 level is increased in diabetic patients. Diabetologia. 1999, Jan. 42(1). 117-8. doi: 10.1007/s001250051124.
- Aukrust P., Halvorsen B., Yndestad A., Ueland T., Øie E., Otterdal K., Gullestad L., Damås J.K. Chemokines and cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2008, Nov. 28(11). 1909-19. doi: 10.1161/ATVBAHA.107.161240.
- Hernández C., Segura R.M., Fonollosa A., Carrasco E., Francisco G., Simó R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet. Med. 2005, Jun. 22(6). 719-22. doi: 10.1111/j.1464-5491.2005.01538.x.
- Maedler K., Santer N.S., Schulthess F.T. The chemokine IP-10 induces beta cell death through TLR-4 signaling. Diabetologia. 2004. 47(Suppl. 1). A-158.
- Shah R., Hinkle C.C., Ferguson J.F., Mehta N.N., Li M., Qu L., Lu Y., Putt M.E., Ahima R.S., Reilly M.P. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes. 2011, May. 60(5). 1512-8. doi: 10.2337/db10-0956.
- Zak K.P., Kondratska I.N., Melnychenko S.V., Popova V.V. The level of circulating IL-16 in the blood of patients with metabolic syndrome and type 2 diabetes. Lik. sprava. 2007. (5–6). 46-9 (in Russian).
- Scherer P.E. The multifaceted roles of adipose tissue-therapeutic targets for diabetes and beyond: The 2015 Banting lecture. Diabetes. 2016, Jun. 65(6). 1452-61. doi: 10.2337/db16-0339.
- Zhao S., Kusminski C.M., Elmquist J.K., Scherer P.E. Leptin: less is more. Diabetes. 2020, May. 69(5). 823-9. doi: 10.2337/dbi19-0018.
- Schmidt M.I., Duncan B.B., Vigo A., Pankow J.S., Couper D., Ballantyne C.M. et al. Leptin and incident type 2 diabetes: risk or protection? Diabetologia. 2006, Sep. 49(9). 2086-96. doi: 10.1007/s00125-006-0351-z.
- Snijder M.B., Heine R.J., Seidell J.C., Bouter L.M., Stehouwer C.D., Nijpels G. et al. Associations of adiponectin levels with incident impaired glucose metabolism and type 2 diabetes in older men and women: the hoorn study. Diabetes Care. 2006, Nov. 29(11). 2498-503. doi: 10.2337/dc06-0952.
- Li V.L., Kim J.T., Long J.Z. Adipose tissue lipokines: recent progress and future directions. Diabetes. 2020, Dec. 69(12). 2541-8. doi: 10.2337/dbi20-0012.
- Kondratska I., Zak K., Mankovsky B. Plasma levels of leptin and adiponectin in patients with type 2 diabetes mellitus (T2DM) with and without metabolic syndrome (MS). J. Diabetes. 2009. 1(Suppl. 1). A-172.
- Кондрацкая И.Н., Зак К.П., Маньковский Б.Н. Уровень циркулирующего лептина в крови у больных с метаболическим синдромом и сахарным диабетом 2-го типа. Український кардіологічний журнал. 2009. 2. 30-3.
- Hanley A.J., Wagenknecht L.E., Norris J.M., Bergman R., Anderson A., Chen Y.I. et al. Adiponectin and the incidence of type 2 diabetes in Hispanics and African Americans: the IRAS Family Study. Diabetes Care. 2011, Oct. 34(10). 2231-6. doi: 10.2337/dc11-0531.106.
- Rasmusen M.S., Lihn A.S., Bruun J.M. Regulation of adiponectin receptor 1 and 2 in human adipose tissue. Diabetologia. 2004. 47(Suppl. 1). A-76.
- Rewers M., Maahs D., Wadwa P. Adiponectin and soluble IL-2 receptor levels predict progression of coronary artery calcification in type 1 diabetes. Diabetologia. 2004. 47(Suppl. 1). A-47.
- Rubin D., Helwig U., Lemke N. Postprandial adiponectin levels after an oral lipid tolerance test versus oral glucose tolerance test and association with parameters of the metabolic syndrome. Diabetologia. 2004. 47(Suppl. 1). A-227.
- Sattar N., McConnachie A., Shaper A.G., Blauw G.J., Buckley B.M., de Craen A.J. et al. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet. 2008, Jun. 7. 371(9628). 1927-35. doi: 10.1016/S0140-6736(08)60602-9.
- Stefan N., Machicao F., Staiger H., Machann J., Schick F., Tschritter O. et al. Polymorphisms in the gene encoding adiponectin receptor 1 are associated with insulin resistance and high liver fat. Diabetologia. 2005, Nov. 48(11). 2282-91. doi: 10.1007/s00125-005-1948-3.
- Wannamethee S.G., Lowe G.D., Rumley A., Cherry L., Whincup P.H., Sattar N. Adipokines and risk of type 2 diabetes in older men. Diabetes Care. 2007, May. 30(5). 1200-5. doi: 10.2337/dc06-2416.
- Li S., Shin H.J., Ding E.L., van Dam R.M. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009, Jul. 8. 302(2). 179-88. doi: 10.1001/jama.2009.976.
- Turer A.T., Scherer P.E. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012, Sep. 55(9). 2319-26. doi: 10.1007/s00125-012-2598-x.
- Tate J., Knuiman M., Davis W.A., Davis T.M.E., Bruce D.G. A comparison of obesity indices in relation to mortality in type 2 diabetes: the Fremantle Diabetes Study. Diabetologia. 2020, Mar. 63(3). 528-36. doi: 10.1007/s00125-019-05057-8.
- Kannel W.B., McGee D.L. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979, May 11. 241(19). 2035-8. doi: 10.1001/jama.241.19.2035.
- Bottle A., Millett C., Khunti K., Majeed A. Trends in cardiovascular admissions and procedures for people with and without diabetes in England, 1996–2005. Diabetologia. 2009, Jan. 52(1). 74-80. doi: 10.1007/s00125-008-1170-1.
- Tuttle K.R., Bakris G.L., Bilous R.W., Chiang J.L., de Boer I.H., Goldstein-Fuchs J. et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014, Oct. 37(10). 2864-83. doi: 10.2337/dc14-1296.
- Cheung N., Mitchell P., Wong T.Y. Diabetic retinopathy. Lancet. 2010, Jul. 10. 376(9735). 124-36. doi: 10.1016/S0140-6736(09)62124-3.
- Antonetti D.A., Klein R., Gardners T.W. Mechanisms of disease: diabetic retinopathy. N. Engl. J. Med. 2012, Mar. 366(13). 1227-39.
- Kumar S., Wilson B., Watson L., Alsop J. Obesity is associated with poorer clinical outcomes following insulin initiation for patients with type 2 diabetes. In: Minutes of the 44th Genral Assembly of the European Association for the Study of Diabetes. Diabetologia. 2009. 52(Suppl. 1). 1-550. doi: 10.1007/s00125-009-1445-1.
- Lichiardopol R., Popescu L.D., Ionescu I., Dovan D., Pencea C. Abdominal obesity in type 1 and type 2 diabetes patients. Diabetologia. 2008. 51(Suppl. 1). S335. doi: 10.1007/s00125-008-1117-6.
- Scheja L., Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019, Sep. 15(9). 507-24. doi: 10.1038/s41574-019-0230-6.
- Cox A.R., Chernis N., Bader D.A., Saha P.K., Masschelin P.M., Felix J.B. et al. STAT1 dissociates adipose tissue inflammation from insulin sensitivity in obesity. Diabetes. 2020, Dec. 69(12). 2630-41. doi: 10.2337/db20-0384.
- Donath M.Y., Dalmas É., Sauter N.S., Böni-Schnetzler M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell. Metab. 2013, Jun. 4. 17(6). 860-72. doi: 10.1016/j.cmet.2013.05.001.
- Kim J.Y., Bacha F., Tfayli H., Michaliszyn S.F., Yousuf S., Arslanian S. Adipose tissue insulin resistance in youth on the spectrum from normal weight to obese and from normal glucose tolerance to impaired glucose tolerance to type 2 diabetes. Diabetes Care. 2019, Feb. 42(2). 265-72. doi: 10.2337/dc18-1178.
- Kopelman P.G. Obesity as a medical problem. Nature. 2000, Apr. 6. 404(6778). 635-43. doi: 10.1038/35007508.
- Harwood H.J. Jr. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology. 2012, Jul. 63(1). 57-75. doi: 10.1016/j.neuropharm.2011.12.010.
- Almuraikhy S., Kafienah W., Bashah M., Diboun I., Jaganjac M., Al-Khelaifi F. et al. Interleukin-6 induces impairment in human subcutaneous adipogenesis in obesity-associated insulin resistance. Diabetologia. 2016, Nov. 59(11). 2406-16. doi: 10.1007/s00125-016-4031-3.
- Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, Jun. 89(6). 2548-56. doi: 10.1210/jc.2004-0395.
- Trayhurn P., Bing C. Appetite and energy balance signals from adipocytes. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006, Jul 29. 361(1471). 1237-49. doi: 10.1098/rstb.2006.1859.
- Nolan J.J., Færch K. Estimating insulin sensitivity and beta cell function: perspectives from the modern pandemics of obesity and type 2 diabetes. Diabetologia. 2012, Nov. 55(11). 2863-7. doi: 10.1007/s00125-012-2684-0.
- Tron'ko N.D., Zak K.P. Obesity and diabetes mellitus. Lik. Sprava. 2013, Dec. (8). 3-21 (in Russian).
- Di Angelantonio E., Bhupathiraju Sh.N., Wormser D., Gao P., Kaptoge S. et al.; Global B.M.I. Mortality Collaboration. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016, Aug. 20. 388(10046). 776-86. doi: 10.1016/S0140-6736(16)30175-1.
- Boutens L., Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016, May. 59(5). 879-94. doi: 10.1007/s00125-016-3904-9.
- McLaughlin T., Abbasi F., Lamendola C., Liang L., Reaven G., Schaaf P., Reaven P. Differentiation between obesity and insulin resistance in the association with C-reactive protein. Circulation. 2002, Dec. 3. 106(23). 2908-12. doi: 10.1161/01.cir.0000041046.32962.86.
- Guarino D., Antonioli L., Fornai M., Pellegrinyi C., Blandizzi C., Anselmino M. et al. Diabetes, obesity and inflammation: persistence of elevated IL-1b after bariatric surgery. In: Abstracts of the 53rd EASD Annual Meeting of the European Association for the Study of Diabetes: Lisbon, Portugal, 11-15 September 2017. Diabetologia. 2017. 60(Suppl. 1). 97. doi: 10.1007/s00125-017-4350-z.
- Somm E., Cettour-Rose P., Asensio C., Charollais A., Klein M., Theander-Carrillo C. et al. Interleukin-1 receptor antagonist is upregulated during diet-induced obesity and regulates insulin sensitivity in rodents. Diabetologia. 2006, Feb. 49(2). 387-93. doi: 10.1007/s00125-005-0046-x.
- Kern P.A., Ranganathan S., Li C., Wood L., Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001, May. 280(5). E745-51. doi: 10.1152/ajpendo.2001.280.5.E745.
- De Carvalho M.H., Colaço A.L., Fortes Z.B. Citocinas, disfunção endotelial e resistência à insulina [Cytokines, endothelial dysfunction, and insulin resistance]. Arq. Bras. Endocrinol. Metabol. 2006, Apr. 50(2). 304-12. Portuguese. doi: 10.1590/s0004-27302006000200016.
- Ryu O., Kim H., Shin D. Effects of exercise on inflammatory markers, insulin resistance, and arterial stiffness. Diabetologia. 2006. 49(Suppl. 1). 470.
- Sumarac-Dumanovic M., Stevanovic D., Ljubic A., Jorga J., Simic M., Stamenkovic-Pejkovic D. et al. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int. J. Obes. (Lond.). 2009. Jan. 33(1). 151-6. doi: 10.1038/ijo.2008.216.
- Laouali N., Mancini F.R., Hajji-Louati M., El Fatouhi D., Balkau B., Boutron-Ruault M.C. et al. Dietary inflammatory index and type 2 diabetes risk in a prospective cohort of 70,991 women followed for 20 years: the mediating role of BMI. Diabetologia. 2019, Dec. 62(12). 2222-32. doi: 10.1007/s00125-019-04972-0.
- Formoso G., Baldassarre M.P.A., Taraborrelli M., D´Adamo M., Di Fulvio P., Di Pietro N. et al. Visceral fat reduction is associated with increased IL 10 levels in obese subjects that underwent caloric restriction. Diabetologia. 2010. 53(Suppl. 1). 783.
- Campbell J.J., Hedrick J., Zlotnik A., Siani M.A., Thompson D.A., Butcher E.C. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998, Jan. 16. 279(5349). 381-4. doi: 10.1126/science.279.5349.381.
- Fernández-Real J.M., Pickup J.C. Innate immunity, insulin resistance and type 2 diabetes. Diabetologia. 2012, Feb. 55(2). 273-8. doi: 10.1007/s00125-011-2387-y.
- Mazurek T. Local epicardial adipose tissue inflammation is associated with serum insulin and insulin resistance in patients with advanced coronary artery disease. Diabetologia. 2004. 47(Suppl. 1). A-31.
- Murdolo G., Hammarstedt A., Sandqvist M., Schmelz M., Herder C., Smith U., Jansson P.A. Monocyte chemoattractant protein-1 in subcutaneous abdominal adipose tissue: characterization of interstitial concentration and regulation of gene expression by insulin. J. Clin. Endocrinol. Metab. 2007, Jul. 92(7). 2688-95. doi: 10.1210/jc.2006-2814.
- Ouwens D.M., Bekaeri M., Lapauw B., Lehr S., Hartwig S., Herzfeld de Wiza D. Sex steroid-induced changes in circulating monocyte chemoattractant protein-1 levels may contribute to metabolic dysfunction in obese men. In: Abstracts of the 48th EASD Annual Meeting: Berlin, Germany, 1–5 October 2012. Diabetologia. 2012. 55(Suppl. 1). A-654. doi: 10.1007/s00125-012-2688-9.
- Fukami A., Adachi H., Yamagishi S-I. A strong association betweem serum level of monocyte chemoattractant protein 1 (MCP-1) and insulin resistant syndrome. Diabetologia. 2007. 50(Suppl. 1). 166.
- Nio Y., Yamauchi T., Iwabu M., Okada-Iwabu M., Funata M., Yamaguchi M. et al. Monocyte chemoattractant protein-1 (MCP-1) deficiency enhances alternatively activated M2 macrophages and ameliorates insulin resistance and fatty liver in lipoatrophic diabetic A-ZIP transgenic mice. Diabetologia. 2012, Dec. 55(12). 3350-8. doi: 10.1007/s00125-012-2710-2.
- Farooqi I.S., O'Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am. J. Clin. Nutr. 2009, Mar. 89(3). 980S-4S. doi: 10.3945/ajcn.2008.26788C.
- Lönnqvist F., Nordfors L., Schalling M. Leptin and its potential role in human obesity. J. Intern. Med. 1999, Jun. 245(6). 643-52. doi: 10.1046/j.1365-2796.1999.00493.x.
- Scott E.M., Grant P.J. Neel revisited: the adipocyte, seasonality and type 2 diabetes. Diabetologia. 2006, Jul. 49(7). 1462-6. doi: 10.1007/s00125-006-0280-x.
- Winkler G., Baranyi E., Melczer Z. Role of TNF-α system and leptin in insulin resistance in patients with gestational diabetes. Diabetologia. 2000. 43(Suppl. 1). A-172.
- Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006, Dec. 14. 444(7121). 860-7. doi: 10.1038/nature05485.
- Dalmas E., Venteclef N., Caer C., Poitou C., Cremer I., Aron-Wisnewsky J. et al. T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes. 2014, Jun. 63(6). 1966-77. doi: 10.2337/db13-1511.
- Hoffstedt J., Andersson D.P., Eriksson Hogling D., Theorell J., Näslund E., Thorell A., Ehrlund A. et al. Long-term protective changes in adipose tissue after gastric bypass. Diabetes Care. 2017, Jan. 40(1). 77-84. doi: 10.2337/dc16-1072.
- Top C., Uslu S.A., Onde M.E. The relationship between body surface area and insulin resistance, serum IL-6 levels in patients with type 2 diabetes. J. Diabetes. 2009. 1(Suppl. 1). A167-8.
- Seyhan A., Nunez-Lopez Yu., Garufi G. Differences in serum cytokine concentrations in lean and obese individuals with prediabetes and type 2 diabetes. Diabetes. 2015. 64(Suppl. 1). A-472.
- Ellingsgaard H., Seelig E., Timper K., Coslovsky M., Soederlund L., Lyngbaek M.P. et al. GLP-1 secretion is regulated by IL-6 signalling: a randomised, placebo-controlled study. Diabetologia. 2020, Feb. 63(2). 362-73. doi: 10.1007/s00125-019-05045-y.
- Grosz A., Nagy E., Halmos T. Inflammatory parametwers in type 2 diabetics and in the metabolic syndrome. Diabetologia. 2006. 49(Suppl. 1). 446.
- Monroy A., Kamath S., Chavez A.O., Centonze V.E., Veerasamy M., Barrentine A. et al. Impaired regulation of the TNF-a converting enzyme/tissue inhibitor of metalloproteinase 3 proteoly–tic system in skeletal muscle of obese type 2 diabetic patients: a new mechanism of insulin resistance in humans. Diabetologia. 2009, Oct. 52(10). 2169-81. doi: 10.1007/s00125-009-1451-3.
- Aas A.-M., Seljeflot I., Birkeland K.I. Effects of lifestyle intervention and insulin treatment on PAI-1, Hs-CRP and TNF-α le–vels in patients with type 2 diabetes. Diabetologia. 2004. 47(Suppl. 1). A-295.
- Zahorska-Markiewicz B., Janowska J., Olszanecka-Glinianowicz M., Zurakowski A. Serum concentrations of TNF-alpha and soluble TNF-alpha receptors in obesity. Int. J. Obes. Relat. Metab. Disord. 2000. Nov. 24(11). 1392-5. doi: 10.1038/sj.ijo.0801398.
- de Carvalho M.H., Colaço A.L., Fortes Z.B. Citocinas, disfunção endotelial e resistência à insulina [Cytokines, endothelial dysfunction, and insulin resistance]. Arq. Bras. Endocrinol. Metabol. 2006, Apr. 50(2). 304-12. Portuguese. doi: 10.1590/s0004-27302006000200016.
- Nikolajczyk B.S., Zhu M., Blanche I.P., Nicholas C., Douglas A. Lauffenburger, Marie E. McDonnell et al. An inflammatory T.cell signature predicts obesity-associated type2 diabetes. Diabetes. 2015. 64(Suppl. 1). A.62 234.
- Baig S., Rizi E.P., Shabeer M., Teo Y., Wee S.Y., Mok S.F. et al. Acute meall challenge and modulation of postprandial immune metabolic response in peripheral blood mononuclear cells, in lean, insulin sensitive and obese, insulin resistant Chinese. Diabetes. 2015. 64(Suppl. 1). A-467. 1805 p.
- Chang Y., Piao S.L., Gao S., Zheng D.M. [Regulatory effects of micronutrient complex on the expression of Th1 and Th2 cytokines in diabetic C57BL mice]. Wei Sheng. Yan. Jiu. 2005, Jan. 34(1). 64-6.
- Erdmann J., Lippl F., Wagenpfeil S., Schusdziarra V. Differential association of basal and postprandial plasma ghrelin with leptin, insulin, and type 2 diabetes. Diabetes. 2005, May. 54(5). 1371-8. doi: 10.2337/diabetes. 54.5.1371.
- Zimmet P.Z., Magliano D.J., De Courten B. Cytokines, advanced glycation end-products, leptin, adiponectin and diabetes: a nested case-control study. Diabetologia. 2008. 51(Suppl. 1). S86.
- Lilja M., Rolandsson O., Norberg M. Leptin independently predicts diabetes in Swedish men. Diabetologia. 2010. 53(Suppl. 1). A-394.
- Kautzky-Willer A., Pacini G., Tura A., Bieglmayer C., Schneider B., Ludvik B. et al. Increased plasma leptin in gestatio–nal diabetes. Diabetologia. 2001, Feb. 44(2). 164-72. doi: 10.1007/s001250051595.
- Kim S.G., Ryu O.H., Kim H.Y., Lee K.W., Seo J.A., Kim N.H. et al. Effect of rosiglitazone on plasma adiponectin le–vels and arterial stiffness in subjects with prediabetes or non-diabetic meta–bolic syndrome. Eur. J. Endocrinol. 2006, Mar. 154(3). 433-40. doi: 10.1530/eje.1.02100.
- Kim J.Y., Ahn S.V., Yoon J.H., Koh S.B., Yoon J., Yoo B.S. et al. Prospective study of serum adiponectin and incident metabo–lic syndrome: the ARIRANG study. Diabetes Care. 2013, Jun. 36(6). 1547-53. doi: 10.2337/dc12-0223.
- Krusinova E., Wohl P., Fejfarova V. Effect of acute hyperinsulinaemia on plasma concentrations of selected cytokine antagonists in type 1 diabetes mellitus. Diabetologia. 2004. 47(Suppl. 1). A-461.
- Ong K.L., Tso A.W., Xu A., Law L.S., Li M., Wat N.M. et al. Evaluation of the combined use of adiponectin and C-reactive protein levels as biomarkers for predicting the deterioration in glycaemia after a median of 5.4 years. Diabetologia. 2011, Oct. 54(10). 2552-60. doi: 10.1007/s00125-011-2227-0.
- Silva-Nunes J., Duarte I., Veiga L. Should hypoadiponectinemia be included as a component of the metabolic syndrome. J. Dia–betes. 2009. 1(Suppl. 1). A-172.
- Menzaghi C., Trischitta V. The adiponectin paradox for all-cause and cardiovascular mortality. Diabetes. 2018, Jan. 67(1). 12-22. doi: 10.2337/dbi17-0016. Erratum in: Diabetes. 2018 Dec. 67(12). 2710.
- Schrieks I.C., Nozza A., Stähli B.E., Buse J.B., Henry R.R., Malmberg K. et al. Adiponectin, free fatty acids, and cardiovascular outcomes in patients with type 2 diabetes and acute coronary syndrome. Diabetes Care. 2018, Aug. 41(8). 1792. doi: 10.2337/dc18-0158.
- McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020, Jun. 19(6). 102537. doi: 10.1016/j.autrev.2020.102537.
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020, Mar. 28. 395(10229). 1033-4. doi: 10.1016/S0140-6736(20)30628-0.
- Crouse A., Grimes T., Li P., Might M., Ovalle F., Shalev A. Metformin use is associated with reduced mortality in a diverse po–pulation with Covid-19 and diabetes. medRxiv. 2020, Jul. 31. doi: 10.1101/2020.07.29.20164020.
- Ugwueze C.V., Ezeokpo B.C., Nnolim B.I., Agim E.A., Anik–po N.C., Onyekachi K.E. COVID-19 and diabetes mellitus: The link and clinical implications. Dubai Diabetes Endocrinol. J. 2020. 26(2). 69-77. doi. 10.1159/000511354.
- Varghese E., Samuel S.M., Liskova A., Kubatka P., Büsselberg D. Diabetes and coronavirus (SARS-CoV-2): Molecular mechanism of Metformin intervention and the scientific basis of drug repurposing. PLoS Pathog. 2021. 17(6). e1009634. https://doi.org/10.1371/journal.ppat. 1009634.

/15.jpg)
/20.jpg)