Международный эндокринологический журнал Том 17, №3, 2021
Вернуться к номеру
Сироватковий рівень WNT-індукованого протеїну 1 як потенційний біомаркер тиреоїдних вузлів
Авторы: Gulhan Duman, Baris Sariakcali
Division of Endocrinology and Metabolism, Department of Internal Medicine, Cumhuriyet University,
Faculty of Medicine, Sivas, Turkey
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
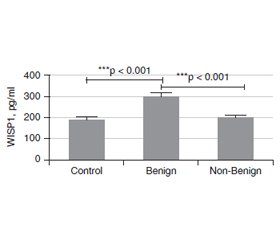
Актуальність. Вузли щитоподібної залози — поширені тиреоїдні захворювання у всьому світі, та їх частота значно зросла за останні десятиліття. Більшість тиреоїдних вузлів зазвичай випадково діагностуються як безсимптомні доброякісні утворення, виявлені методами візуалізації, проведеними з причин, не пов’язаних із захворюваннями щитоподібної залози. Метою даного дослідження було встановити значення рівня WNT-індукованого протеїну 1 (WISP1) у сироватці крові як допоміжного біомаркера для проведення диференціальної діагностики доброякісних та недоброякісних вузлів щитоподібної залози. Матеріали та методи. У дослідженні брали участь 89 пацієнтів, яким проведено тонкоголкову аспіраційну біопсію, та 43 особи контрольної групи. Серед обстежених жінки становили 72,7 % та 27,3 % — чоловіки. Вони були розподілені на дві групи відповідно до цитологічної оцінки Bethesda: доброякісні (Bethesda 2) та недоброякісні (Bethesda 3–6) утворення. Рівень WISP1 у сироватці крові вимірювали методом імуноферментного аналізу. Результати. У групі з доброякісними вузлами (Bethesda 2) були 58 (43,9 %) пацієнтів, та 31 (23,5 %) — у групі з недоброякісними (Bethesda 3–6) вузлами. Установлено, що розмір утворень був більшим у групі з недоброякісними вузлами, ніж у групі з доброякісними (p = 0,006). Рівень WISP1 у сироватці крові в групі хворих із доброякісними вузлами (Bethesda 2) був вірогідно вищим, ніж у групі з недоброякісними утвореннями (Bethesda 3–6) та осіб контрольної групи (p < 0). Різниця між хворими з доброякісними та недоброякісними вузлами відповідно до їх ехогенності була значущою (р < 0,05). У групі з доброякісними вузлами 76,9 % утворень мали змішану ехогенність, 76,7 % — були ізоехогенними, 68,4 % — ізогіпоехогенними та 35,7 % — гіпоехогенними. У хворих із доброякісними вузлами відзначалися найвища гіпоехогенність (64,3 %) і найменша змішана ехогенність (23,1 %). Не встановлено зв’язку між рівнями WISP1 та ехогенністю за допомогою критерію Kruskal-Wallis. Висновки. Згідно з результатами проведеного дослідження, вимірювання WISP1 у сироватці крові дозволяє отримати додаткову інформацію при диференціально-діагностичному аналізі пацієнтів із вузлами щитоподібної залози. Більш високий рівень сироваткового WISP1 дозволяє підтвердити цитологічний діагноз у хворих з доброякісними вузлами щитоподібної залози (Bethesda 2).
Background. Thyroid nodule (TN) is a common thyroid disease worldwide, and it has increased significantly last decades. Most TNs are usually incidental findings of asymptomatic, benign lesions discovered by imaging modalities performed for reasons unrelated to thyroid diseases. The purpose of this study was to investigate the value of serum WNT-induced secreted protein 1 (WISP1) level as a supporting biomarker to perform differential diagnosis of benign and non-benign thyroid nodules. Materials and methods. The study was completed with the 89 patients undergone fine needle aspiration biopsy and 43 controls. The patients were composed of 96 (72.7 %) females and 36 (27.3 %) males. And they were divided into 2 group according to the Bethesda cytological evaluation as Benign (Bethesda 2) and Non-Benign (Bethesda 3–6) groups. Their serum WISP1 levels were measured by an ELISA method. Results. There were 58 (43.9 %) patients in Benign (Bethesda 2) and 31 (23.5 %) in non-Benign (Bethesda 3–6) groups. In the contrary nodule size was bigger in the Non-benign group than that benign group (p = 0.006). The serum WISP1 level in the Benign (Bethesda 2) group was significantly higher than that in the and Non-Benign (Bethesda 3–6) group, and controls (p < 0). The difference between benign and non-benign group accordingly to their echogenicitiy was significant (p < 0.05). In benign group there was 76.9 % mixed echoic nodules, 76.7 % isoechoic nodules 68.4 % isohypoechoic nodules and 35.7 % hypoechoic nodules. In the non-benign group, the highest hypoechoic echo (64.3 %), the least mixed echo (23.1 %), while in the benign group, the most mixed echo (76.9 %), the least hypoechoic echo (35.7 %) was present. There was no relation between WISP1 levels and echogenicity with Kruskal-Wallis H test. Conclusions. According to the preliminary results of current study, addition of serum WISP1 measurement to the differential diagnostic work-up of thyroid nodules patients may provide supportive information. In thyroid nodules patients with Benign (Bethesda 2) category of cytological evaluation, a higher level of serum WISP1 may support cytological diagnosis.
вузли щитоподібної залози; ультразвукова діагностика; тонкоголкова аспіраційна біопсія; WISP1
thyroid nodule; thyroid ultrasonography; fine-needle aspiration biopsy; WISP1
Introduction
Materials and Methods
Results
/46.jpg)
Discussion
Conclusions
- Al Dawish M.A., Robert A.A., Muna A., Eyad A., Al Ghamdi A., Al Hajeri K., Thabet M.A., Braham R. Bethesda System for Reporting Thyroid Cytopathology: A three-year study at a tertiary care referral center in Saudi Arabia. World J. Clin. Oncol. 2017. 8(2). 151-157. doi: 10.5306/wjco.v8.i2.151.
- Janczak D., Pawlowski W., Dorobisz T., Janczak D., Dorobisz K., Leśniak M., Ziomek A., Chabowski M. An evaluation of the diagnostic efficacy of fine needle aspiration biopsy in patients operated for a thyroid nodular goiter. Onco Targets Ther. 2016. 9. 5819-5823. doi: 10.2147/OTT.S111275.
- Diana S.D., Hossein G. Epidemiology of thyroid nodules. Best Practice & Research: Clinical Endocrinology & Metabolism 2008. 22(6). 901-911. doi: 10.1016/j.beem.2008.09.019.
- Bomeli S.R., LeBeau S.O., Ferris R.L. Evaluation of a thyroid nodule. Otolaryngol Clin. North Am. 2010. 43(2). 229-238. doi: 10.1016/j.otc.2010.01.002.
- Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E., Pacini F. et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016. 26(1). 1-133. doi: 10.1089/thy.2015.0020.
- Yang J., Schnadig V., Logrono R., Wasserman P.G. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007. 111(5). 306-315. doi: 10.1002/cncr.22955.
- Baloch Z.W., LiVolsi V.A., Asa S.L., Rosai J., Merino M.J., Randolph G., Vielh P. et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn. Cytopathol. 2008. 36(6). 425-437. doi: 10.1002/dc.20830.
- Meko J.B., Norton J.A. Large cystic/solid thyroid nodules: a potential false-negative fine-needle aspiration. Surgery. 1995. 118. 996-1003. doi: 10.1016/S0039-6060(05)80105-9.
- Shin J.J., Caragacianu D., Randolph G.W. Impact of thyroid nodule size on prevalence and post-test probability of malignancy: a systematic review. Laryngoscope. 2015. 125(1). 263-272. doi: 10.1002/lary.24784.
- Hsiao S.J., Nikiforov Y.E. Molecular approaches to thyroid cancer diagnosis. Endocr. Relat. Cancer. 2014. 21(5). 301-313. doi: 10.1530/ERC-14-0166.
- Özdamar O.İ., Acar G.Ö., Özen F., Zenginkinet T. Assessment of BRAF V600E, KRAS, NRAS and EGFR mutations in Papillary Thyroid Carcinoma and Hashimoto Thyroiditis. Clinical Research. ENT Updates 2020. 10(2). 300-305. doi: 10.32448/entupdates.711666.
- Wang W., Chang J., Jia B., Liu J. The Blood Biomarkers of Thyroid Cancer. Cancer Manag. Res. 2020. 12. 5431-5438. doi: 10.2147/CMAR.S261170.
- Chiang K.C., Yeh C.N., Chung L.C. et al. WNT-1 inducible signaling pathway protein-1 enhances growth and tumorigenesis in human breast cancer. Sci. Rep. 2015. 5. 8686. doi: 10.1038/srep08686.
- Deng W., Fernandez A., McLaughlin S.L., Klinke D.J. 2nd. WNT1-inducible signaling pathway protein 1 (WISP1/CCN4) stimulates melanoma invasion and metastasis by promoting the epithelial-mesenchymal transition. J. Biol. Chem. 2019. 294(14). 5261-5280. doi: 10.1074/jbc. RA118.006122.
- Jia S., Qu T., Feng M. et al. Association of Wnt1-inducible signaling pathway protein-1 with the proliferation, migration and invasion in gastric cancer cells. Tumour Biol. 2017. 39(6). 1010428317699755. doi: 10.1177/1010428317699755.
- Evranos B., Polat S.B., Baser H., Ozdemir D., Kilicarslan A., Yalcin A., Ersoy R., Cakir B. Bethesda classification is a valuable guide for fine needle aspiration reports and highly predictive especially for diagnosing aggressive variants of papillary thyroid carcinoma. Cytopathology. 2017. 28(4). 259-267. doi: 10.1111/cyt.12384.
- Arora N., Scognamiglio T., Zhu B., Fahey T.J. 3rd. Do benign thyroid nodules have malignant potential? An evidence-based review. World J. Surg. 2008. 32(7). 1237-1246. doi: 10.1007/s00268-008-9484-1.
- Russ G., Bonnema S.J., Erdogan M.F., Durante C., Ngu R., Leenhardt L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017. 6(5). 225-237. doi: 10.1159/000478927.
- Bongiovanni M., Spitale A., Faquin W.C., Mazzucchelli L., Baloch Z.W. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol. 2012. 56(4). 333-339. doi: 10.1159/000339959.
- Ha E.J., Baek J.H., Na D.G. Risk Stratification of Thyroid Nodules on Ultrasonography: Current Status and Perspectives. Thyroid. 2017. 27(12). 1463-1468. doi: 10.1089/thy.2016.0654.
- Meng Z., Tan J., Zhang G. et al. Evaluation of serum midkine as a biomarker in differentiated thyroid cancer. Life Sci. 2015. 130. 18-24. doi: 10.1016/j. lfs.2015.02.028.
- Hu Z., Zhao P., Zhang K., Zang L., Liao H., Ma W. Evaluation of Serum Vascular Adhesion Protein-1 as a Potential Biomarker in Thyroid Cancer. Int. J. Endocrinol. 2016. 2016. 1-7. doi: 10.1155/2016/6312529.
- Edmund S. Cibas and Syed Z. Ali. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017. 27(11). doi: 10.1089/thy.2017.0500.
- Kleiman D.A., Beninato T., Soni A., Shou Y., Zarnegar R., Fahey T.J. 3rd. Does bethesda category predict aggressive features in malignant thyroid nodules? Ann. Surg. Oncol. 2013. 20(11). 3484-3490. doi: 10.1245/s10434-013-3076-5.
- Pennica D., Swanson T.A., Welsh J.W. et al. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc. Natl Acad. Sci USA. 1998. 95(25). 14717-14722. doi: 10.1073/pnas.95.25.14717.
- Xu L., Corcoran R.B., Welsh J.W., Pennica D., Levine A.J. WISP1 is a Wnt-1- and beta-catenin-responsive oncogene. Genes. Dev. 2000. 14(5). 585-595. PMID: 10716946.
- Kenneth M. WISP1: Clinical Insights for a Proliferative and Restorative Member of the CCN Family. Curr. Neurovasc. Res. 2014. 11(4). 378-389. doi: 10.2174/1567202611666140912115107.142–146.
- Gurbuz I., Chiquet-Ehrismann R. CCN4/WISP1 (WNT1 inducible signaling pathway protein 1): a focus on its role in cancer. Int. J. Biochem. Cell. Biol. 2015. 62. 142-146. doi: 10.1016/j.biocel.2015.03.007.
- Jianghong W., Ziwen L., Hong C., Chunyan D., Xiaowen L. Identification of WISP1 as a novel oncogene in glioblastoma. Oncotarget. 2015. 7(31). 49834-49847. doi: 10.18632/oncotarget.10486.
- Taghavi A., Akbari M.E., Hashemi-Bahremani M., Nafissi N., Khalilnezhad A., Poorhosseini S.M., Hashemi-Gorji F., Yassaee V.R. Gene expression profiling of the 8q22-24 position in human breast cancer: TSPYL5, MTDH, ATAD2 and CCNE2 genes are implicated in oncogenesis, while WISP1 and EXT1 genes may predict a risk of metastasis. Oncol. Lett. 2016. 12(5). 3845-3855. doi: 10.3892/ol.2016.5218.
- Nagai Y., Watanabe M., Ishikawa S., Karashima R., Kurashige J., Iwagami S., Iwatsuki M. et al. Clinical significance of Wnt-induced secreted protein-1 (WISP1/CCN4) in esophageal squamous cell carcinoma. Anticancer Res. 2011. 31(3). 991-997. PMID: 21498727.
- Davies S.R., Davies M.L., Sanders A., Parr C., Torkington J., Jiang W.G. Differential expression of the CCN family member WISP1, WISP-2 and WISP-3 in human colorectal cancer and the prognostic implications. Int. J. Oncol. 2010. 36. 1129-1136. doi: 10.3892/ijo_00000595.
- Shao H., Cai L., Grichnik J.M., Livingstone A.S., Velazquez O.C., Liu Z.J. Activation ofNotch1 signaling in stromal fibroblasts inhibits melanoma growth by upregulating WISP1. Oncogene. 2011. 30. 4316-4326. doi: 10.1038/onc.2011.142.
- Soon L.L., Yie T.-A., Shvarts A., Levine A.J., Su F., Tchou-Wong K.-M. Overexpression of WISP1 Down-regulated Motility and Invasion of Lung Cancer Cells through Inhibition of Rac Activation. J. Biol. Chem. 2003. 278(13). 11465-11470. doi: 10.1074/jbc.M210945200.
- Mueller M.M., Fusenig N.E. Friends or foes — bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer. 2004. 4(11). 839-849. doi: 10.1038/nrc1477. PMID: 15516957.
- Rupp C., Scherzer M., Rudisch A. et al. IGFBP7, a novel tumor stroma marker, with growth-promoting effects in colon cancer through a paracrine tumorstroma interaction. Oncogene. 2015. 34. 815-825. doi: 10.1038/onc.2014.18.
- Bauer M., Su G., Casper C., He R., Rehrauer W., Friedl A. Heterogeneity of gene expression in stromal fibroblasts of human breast carcinomas and normal breast. Oncogene. 2010. 29. 1732-1740. doi: 10.1038/onc.2009.463.
- Tanaka S., Sugimachi K., Kameyama T., Maehara S., Shirabe K., Shimada M., Wands J.R., Maehara Y. Human WISP1v, a member of the CCN family, is associated with invasive cholangiocarcinoma. Hepatology. 2003. 37(5). 1122-1129. doi: 10.1053/jhep.2003.50187.
- Ono M., Inkson C.A., Sonn R., Kilts T.M., de Castro L.F., Maeda A., Fisher L.W. et al. WISP1/CCN4: a potential target for inhibiting prostate cancer growth and spread to bone. PLoS One. 2013. 8(8). e71709. doi: 10.1371/journal.pone.0071709.
- Feng M., Jia S. Dual effect of WISP1 in diverse pathological processes. Chinese J. Cancer Res. 2016. 28(6). 553-560. doi: 10.21147/j. issn.1000-9604.2016.06.01.

/44.jpg)
/45.jpg)