Журнал «Здоровье ребенка» Том 16, №2, 2021
Вернуться к номеру
Значення αβТ-клітин у розвитку метазапалення жирової тканини при ожирінні
Авторы: Абатуров О.Є., Нікуліна А.O.
Дніпровський державний медичний університет, м. Дніпро, Україна
Рубрики: Педиатрия/Неонатология
Разделы: Справочник специалиста
Версия для печати
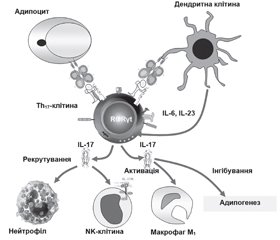
У літературному огляді наведені сучасні уявлення стосовно ролі αβТ-клітин, які представлені основними групами: CD4+-, CD8+Т-лімфоцитами та двічі негативними CD4–CD8–-Т- і двічі позитивними CD4+CD8+-Т-клітинами адаптивної імунної системи організму, у розвитку метазапалення жирової тканини при ожирінні. Відповідно до сучасної концепції ожиріння асоціюється з гіпертрофією адипоцитів, порушенням адипогенезу, розвитком метазапалення жирової тканини, що відрізняється в ранній адаптивній фазі швидкою реактивацією Т-клітин пам’яті. Фізичний зв’язок клітин, що виконують функцію накопичення енергії, із клітинами імунологічної пам’яті являє собою особливий механізм виживання організму в періоди дефіциту харчування. Надлишок жирової тканини і поява прозапальних гіпертрофованих адипоцитів змінюють спектр і співвідношення продукованих адипокінів і цитокінів, що призводить до рекрутування й активації прозапальних імуноцитів і виснаження пулу протизапальних клітин вродженої й адаптивної імунної системи в жировій тканині. Зміни представництва резидентних рекрутованих імунних клітин, у жировій тканині під час ожиріння характеризуються акумуляцією прозапальних клітин (нейтрофіли, M1-Mϕ, тучні, NK-, Th1-, Th17-, Th22-клітини) і виснаженням регуляторних і протизапальних популяцій (еозинофіли, M2-Mϕ, ICL2, iNKT, γδT-, Th2-, Treg-, B1-клітини). Надлишкова продукція IFN-γ і TNF-α призводить до розвитку інсулінорезистентності, а IL-17 — до деградації екстрацелюлярного матриксу жирової тканини і порушення адипогенезу. При ожирінні CD8+-Т-клітини і M1-Mϕ елімінують гіпертрофовані адипоцити. Зниження активності Treg-клітин збільшує прозапальний потенціал жирової тканини. Незважаючи на досягнуті успіхи в розкритті ролі адаптивної імунної системи при розвитку ожиріння, до сьогодні не ідентифіковані асоційовані з ожирінням антигени жирової тканини, механізми їх генерації, не визначена роль більшості тканиноспецифічних алармінів; не відомі механізми, що визначають час, послідовність й активність рекрутування різних типів імуноцитів. Наведені дані сучасних досліджень імунопатогенезу метазапалення жирової тканини і створення лікарських засобів, що дозволять підвищити ефективність й індивідуалізувати протизапальну терапію у хворих з ожирінням.
The literature review presents modern ideas about the role of αβ T cells, which are represented by the main groups: CD4+, CD8+ T lymphocytes and double-negative CD4–CD8– T and double-positive CD4+CD8+ T cells of the adaptive immune system of the organism in the development of metainflammation of adipose tissue in obesity. According to the modern concept, obesity is associated with adipocyte hypertrophy, impaired adipogenesis, the development of metainflammation of adipose tissue that in the early adaptive phase is characterized by the rapid reactivation of memory T cells. The physical connection of cells performing the function of energy storage with immunological memory cells is a special mechanism for the body’s survival during periods of nutritional deficiency. Excess adipose tissue and the appearance of pro-inflammatory hypertrophied adipocytes change the spectrum and ratio of produced adipokines and cytokines, which leads to the recruitment and activation of pro-inflammatory immunocytes and depletion of the pool of anti-inflammatory cells of the innate and adaptive immune system in adipose tissue. Changes in the representation of resident recruited immune cells in adipose tissue during obesity is characterized by the accumulation of pro-inflammatory cells (neutrophils, M1 Mϕ, mast cells, NK, Th1, Th17, Th22 cells) and depletion of regulatory and anti-inflammatory populations (eosinophils, M2 Mϕ, ILC2, iNKT, γδ T, Th2, Treg, B1 cells). Excessive production of interferon γ and tumor necrosis factor α leads to the development of insulin resistance, and interleukin 17 — to degradation of the extracellular matrix of adipose tissue and impaired adipogenesis. In obesity, CD8+ T cells and M1 Mϕ macrophages eliminate hypertrophied adipocytes. A decrease in the activity of Treg cells increases the pro-inflammatory potential of adipose tissue. Despite the progress achieved in disclosing the role of the adaptive immune system in the development of obesity, the antigens associated with adipose tissue, the mechanisms of their generation have not been identified so far, the role of most tissue-specific alarmins has not been determined; the mechanisms that determine the timing, sequence and activity of recruitment of various types of immunocytes are not known. The data of modern studies on the immunopathogenesis of metainflammation of adipose tissue and the creation of drugs that will improve the efficiency and individualize anti-inflammatory therapy in obese patients are presented.
адаптивна імунна система; Т-лімфоцити; метазапалення; ожиріння; діти
adaptive immune system; T-lymphocytes; metainflammation; obesity; children
Вступ
1. Коротка характеристика αβТ-клітин
2. CD4+-Т-клітини
3. Цитотоксичні CD8+-Т-клітини
Висновок
- Абатуров А.Е. Метаболический синдром у детей (лекция). Таврический медико-биологический вестник. 2007. Т. 10. С. 57-65.
- Абатуров А.Е. Особенности метаболического синдрома у детей. Дитячий лікар. 2011. № 4(11). С. 54-61.
- Ahmed M., Gaffen S.L. IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev. 2010. 21(6). 449-453. doi: 10.1016/j.cytogfr.2010.10.005.
- Asghar A., Sheikh N. Role of immune cells in obesity induced low grade inflammation and insulin resistance. Cell. Immunol. 2017. 315. 18-26. doi: 10.1016/j.cellimm.2017.03.001.
- Bagheri H., Pourhanifeh M.H., Derakhshan M. et al. CXCL-10: a new candidate for melanoma therapy? Cell. Oncol. (Dordr). 2020. 43(3). 353-365. doi: 10.1007/s13402-020-00501-z.
- Bernardini N., Skroza N., Tolino E. et al. IL-17 and its role in inflammatory, autoimmune, and oncological skin diseases: state of art. Int. J. Dermatol. 2020. 59(4). 406-411. doi: 10.1111/ijd.14695.
- Bertola A., Ciucci T., Rousseau D. et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012. 61(9). 2238-2247. doi: 10.2337/db11-1274.
- Busch D.H., Fräßle S.P., Sommermeyer D., Buchholz V.R., Riddell S.R. Role of memory T cell subsets for adoptive immunotherapy. Semin Immunol. 2016. 28(1). 28-34. doi: 10.1016/j.smim.2016.02.001.
- Caër C., Rouault C., Le Roy T. et al. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci. Rep. 2017. 7(1). 3000. Published 2017, Jun 7. doi: 10.1038/s41598-017-02660-w.
- Cardellini S., Socci C., Bissolati M. et al. Enrichment of Tc1 cells and T cell resistance to suppression are associated with dysglycemia in the visceral fat in human obesity. BMJ Open Diabetes Res. Care. 2020. 8(1). e000772. doi: 10.1136/bmjdrc-2019-000772.
- Cayrol C., Girard J.P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018. 281(1). 154-168. doi: 10.1111/imr.12619.
- Chang S.H. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch. Pharm. Res. 2019. 42(7). 549-559. doi: 10.1007/s12272-019-01146-9.
- Chehimi M., Vidal H., Eljaafari A. Pathogenic Role of IL-17-Producing Immune Cells in Obesity, and Related Inflammatory Diseases. J. Clin. Med. 2017. 6(7). 68. Published 2017, Jul 14. doi: 10.3390/jcm607006.
- Chen X., Wang S., Huang Y. et al. Obesity Reshapes Visceral Fat-Derived MHC I Associated-Immunopeptidomes and Generates Antigenic Peptides to Drive CD8+ T Cell Responses [published online ahead of print, 2020 Mar 13]. iScience. 2020. 23(4). 100977. doi: 10.1016/j.isci.2020.100977.
- Chng M.H., Alonso M.N., Barnes S.E., Nguyen K.D., Engleman E.G. Adaptive Immunity and Antigen-Specific Activation in Obesity-Associated Insulin Resistance. Mediators Inflamm. 2015. 2015. 593075. doi: 10.1155/2015/593075.
- Cho K.W., Morris D.L., DelProposto J.L. et al. An MHC II-dependent activation loop between adipose tissu.e macrophages and CD4+ T cells controls obesity-induced inflammation. Cell. Rep. 2014. 9(2). 605-617. doi: 10.1016/j.celrep.2014.09.004.
- Cipolletta D., Cohen P., Spiegelman B.M., Benoist C., Mathis D. Appearance and disappearance of the mRNA signature characteristic of Treg cells in visceral adipose tissue: age, diet, and PPARγ effects. Proc. Natl Acad. Sci. USA. 2015. 112(2). 482-487. doi: 10.1073/pnas.1423486112.
- Cipolletta D., Feuerer M., Li A. et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012. 486(7404). 549-553. doi: 10.1038/nature11132.
- Dalmas E., Venteclef N., Caer C. et al. T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes. 2014. 63(6). 1966-1977. doi: 10.2337/db13-1511.
- de Souza A.W., Mesquita Júnior D., Araújo J.A. et al. Immune system: part III. The delicate balance of the immune system between tolerance and autoimmunity. Rev. Bras. Reumatol. 2010. 50(6). 665-679.
- Deiuliis J., Shah Z., Shah N. et al. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS One. 2011. 6(1). e16376. Published 2011, Jan 26. doi: 10.1371/journal.pone.0016376.
- Deng J., Yu X.Q., Wang P.H. Inflammasome activation and Th17 responses. Mol. Immunol. 2019. 107. 142-164. doi: 10.1016/j.molimm.2018.12.024.
- Deng T., Lyon C.J., Minze L.J. et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell. Metab. 2013. 17(3). 411-422. doi: 10.1016/j.cmet.2013.02.009.
- Deng T., Liu J., Deng Y. et al. Adipocyte adaptive immunity mediates diet-induced adipose inflammation and insulin resistance by decreasing adipose Treg cells. Nat. Commun. 2017. 8. 15725. https://doi.org/10.1038/ncomms15725.
- Doedens A.L., Phan A.T., Stradner M.H. et al. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat. Immunol. 2013. 14(11). 1173-1182. doi: 10.1038/ni.2714.
- Duffaut C., Zakaroff-Girard A., Bourlier V. et al. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb. Vasc. Biol. 2009. 29(10). 1608-1614. doi: 10.1161/ATVBAHA.109.192583.
- Egeberg A., Gisondi P., Carrascosa J.M., Warren R.B., Mrowietz U. The role of the interleukin-23/Th17 pathway in cardiometabolic comorbidity associated with psoriasis [published online ahead of print, 2020 Feb 5]. J. Eur. Acad. Dermatol. Venereol. 2020. doi: 10.1111/jdv.16273. doi:10.1111/jdv.16273.
- Endo Y., Asou H.K., Matsugae N. et al. Obesity Drives Th17 Cell Differentiation by Inducing the Lipid Metabolic Kinase, ACC1. Cell. Rep. 2015. 12(6). 1042-1055. doi: 10.1016/j.celrep.2015.07.014.
- Endo Y., Yokote K., Nakayama T. The obesity-related pathology and Th17 cells. Cell. Mol. Life Sci. 2017. 74(7). 1231-1245. doi: 10.1007/s00018-016-2399-3.
- Engin A. Adipose Tissue Hypoxia in Obesity and Its Impact on Preadipocytes and Macrophages: Hypoxia Hypothesis. Adv. Exp. Med. Biol. 2017. 960. 305-326. doi: 10.1007/978-3-319-48382-5_13.
- Esser N., Paquot N., Scheen A.J. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin. Investig. Drugs. 2015. 24(3). 283-307. doi: 10.1517/13543784.2015.974804.
- Fabbrini E., Cella M., McCartney S.A. et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013. 145(2). 366-374.e743. doi: 10.1053/j.gastro.2013.04.010.
- Ferrante A.W. Jr. The immune cells in adipose tissue. Diabetes Obes. Metab. 2013. 15, Suppl 3(03). 34-38. doi: 10.1111/dom.12154.
- Ferreira L.M.R., Muller Y.D., Bluestone J.A., Tang Q. Next-generation regulatory T cell therapy. Nat. Rev. Drug. Discov. 2019. 18(10). 749-769. doi: 10.1038/s41573-019-0041-4.
- Feuerer M., Herrero L., Cipolletta D. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009. 15(8). 930-939. doi: 10.1038/nm.2002.
- Frydrych L.M., Bian G., O’Lone D.E., Ward P.A., Delano M.J. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J. Leukoc. Biol. 2018. 104(3). 525-534. doi: 10.1002/JLB.5VMR0118-021RR.
- Furue M., Furue K., Tsuji G., Nakahara T. Interleukin-17A and Keratinocytes in Psoriasis. Int. J. Mol. Sci. 2020. 21(4). 1275. Published 2020, Feb 13. doi: 10.3390/ijms21041275.
- Gagliani N., Huber S. Basic Aspects of T Helper Cell Differentiation. Methods Mol. Biol. 2017. 1514. 19-30. doi: 10.1007/978-1-4939-6548-9_2.
- Georgiev P., Charbonnier L.M., Chatila T.A. Regulatory T Cells: the Many Faces of Foxp3. J. Clin. Immunol. 2019. 39(7). 623-640. doi: 10.1007/s10875-019-00684-7.
- Golubovskaya V., Wu L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers (Basel). 2016. 8(3). 36. Published 2016, Mar 15. doi: 10.3390/cancers8030036.
- Gregory J.W. Prevention of Obesity and Metabolic Syndrome in Children. Front Endocrinol (Lausanne). 2019. 10. 669. Published 2019, Oct 1. doi: 10.3389/fendo.2019.00669.
- Gyllenhammer L.E., Lam J., Alderete T.L. et al. Lower omental t-regulatory cell count is associated with higher fasting glucose and lower β-cell function in adults with obesity. Obesity (Silver Spring). 2016. 24(6). 1274-1282. doi: 10.1002/oby.21507.
- Han S.J., Glatman Zaretsky A., Andrade-Oliveira V. et al. White Adipose Tissue Is a Reservoir for Memory T Cells and Promotes Protective Memory Responses to Infection. Immunity. 2017. 47(6). 1154-1168.e6. doi: 10.1016/j.immuni.2017.11.009.
- Iqbal Z., Tadi P. Histology, Cytotoxic T Cells (CD8+). In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2020.
- Jia L., Wu C. The biology and functions of Th22 cells. Adv. Exp. Med. Biol. 2014. 841. 209-230. doi: 10.1007/978-94-017-9487-9_8.
- Jiang E., Perrard X.D., Yang D. et al. Essential role of CD11a in CD8+ T-cell accumulation and activation in adipose tissue. Arterioscler Thromb. Vasc. Biol. 2014. 34(1). 34-43. doi: 10.1161/ATVBAHA.113.302077.
- Joetham A., Schedel M., Ning F., Wang M., Takeda K., Gelfand E.W. Dichotomous role of TGF-β controls inducible regulatory T-cell fate in allergic airway disease through Smad3 and TGF-β-activated kinase 1. J. Allergy Clin. Immunol. 2020. 145(3). 933-946. e4. doi: 10.1016/j.jaci.2019.09.032.
- Jung C., Lichtenauer M., Strodthoff D. et al. Alterations in systemic levels of Th1, Th2, and Th17 cytokines in overweight adolescents and obese mice. Pediatr. Diabetes. 2017. 18(8). 714-721. doi: 10.1111/pedi.12435.
- Kalathookunnel Antony A., Lian Z., Wu H. T Cells in Adipose Tissue in Aging. Front. Immunol. 2018. 9. 2945. Published 2018, Dec 12. doi: 10.3389/fimmu.2018.02945.
- Kälin S., Becker M., Ott V.B. et al. A Stat6/Pten Axis Links Regulatory T Cells with Adipose Tissue Function. Cell. Metab. 2017. 26(3). 475-492. e7. doi: 10.1016/j.cmet.2017.08.008.
- Kang K., Reilly S.M., Karabacak V. et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell. Metab. 2008. 7(6). 485-495. doi: 10.1016/j.cmet.2008.04.002.
- Kawanishi N., Mizokami T., Yano H., Suzuki K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med. Sci. Sports Exerc. 2013. 45(9). 1684-1693. doi: 10.1249/MSS.0b013e31828ff9c6.
- Khan I.M., Dai Perrard X.Y., Perrard J.L. et al. Attenuated adipose tissue and skeletal muscle inflammation in obese mice with combined CD4+ and CD8+ T cell deficiency. Atherosclerosis. 2014. 233(2). 419-428. doi: 10.1016/j.atherosclerosis.2014.01.011.
- Khan S., Chan Y.T., Revelo X.S., Winer D.A. The Immune Landscape of Visceral Adipose Tissue During Obesity and Aging. Front Endocrinol (Lausanne). 2020. 11. 267. Published 2020, May 15. doi: 10.3389/fendo.2020.00267.
- Kim E.Y., Noh H.M., Choi B. et al. Interleukin-22 Induces the Infiltration of Visceral Fat Tissue by a Discrete Subset of Duffy Antigen Receptor for Chemokine-Positive M2-Like Macrophages in Response to a High Fat Diet. Cells. 2019. 8(12). 1587. Published 2019, Dec 6. doi: 10.3390/cells8121587.
- Kim K.Y., Jeong H.J., Kim H.M. The role of T-bet in obesity: lack of T-bet causes obesity in male mice. J. Nutr. Biochem. 2013. 24(1). 240-247. doi: 10.1016/j.jnutbio.2012.05.010.
- Kintscher U., Hartge M., Hess K. et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb. Vasc. Biol. 2008. 28(7). 1304-1310. doi: 10.1161/ATVBAHA.108.165100.
- Kolb R., Sutterwala F.S., Zhang W. Obesity and cancer: inflammation bridges the two. Curr. Opin. Pharmacol. 2016. 29. 77-89. doi: 10.1016/j.coph.2016.07.005.
- Kolodin D., van Panhuys N., Li C. et al. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell. Metab. 2015. 21(4). 543-557. doi: 10.1016/j.cmet.2015.03.005.
- Konjar Š., Veldhoen M. Dynamic Metabolic State of Tissue Resident CD8 T Cells. Front. Immunol. 2019. 10. 1683. Published 2019, Jul 17. doi: 10.3389/fimmu.2019.01683.
- Kraszula L., Jasińska A., Eusebio M., Kuna P., Głąbiński A., Pietruczuk M. Evaluation of the relationship between leptin, resistin, adiponectin and natural regulatory T cells in relapsing-remitting multiple sclerosis. Neurol. Neurochir. Pol. 2012. 46(1). 22-28. doi: 10.5114/ninp.2012.27211.
- Kucharska A.M., Pyrżak B., Demkow U. Regulatory T Cells in Obesity. Adv. Exp. Med. Biol. 2015. 866. 35-40. doi: 10.1007/5584_2015_147.
- Kuryłowicz A., Koźniewski K. Anti-Inflammatory Strategies Targeting Metaflammation in Type 2 Diabetes. Molecules. 2020. 25(9). 2224. Published 2020, May 9. doi: 10.3390/molecules25092224.
- Le Jemtel T.H., Samson R., Milligan G., Jaiswal A., Oparil S. Visceral Adipose Tissue Accumulation and Residual Cardiovascular Risk. Curr. Hypertens Rep. 2018. 20(9). 77. Published 2018, Jul 10. doi: 10.1007/s11906-018-0880-0.
- Lee Y., Kuchroo V. Defining the functional states of Th17 cells. F1000Res. 2015; 4 (F1000 Faculty Rev). 132. Published 2015, May 28. doi: 10.12688/f1000research.6116.1.
- Li C., DiSpirito J.R., Zemmour D. et al. TCR Transgenic Mice Reveal Stepwise, Multi-site Acquisition of the Distinctive Fat-Treg Phenotype. Cell. 2018. 174(2). 285-299. e12. doi: 10.1016/j.cell.2018.05.004.
- Li C., Spallanzani R.G., Mathis D. Visceral adipose tissue Tregs and the cells that nurture them. Immunol. Rev. 2020. 295(1). 114-125. doi: 10.1111/imr.12850.
- Li J., Tan J., Martino M.M., Lui K.O. Regulatory T-Cells: Potential Regulator of Tissue Repair and Regeneration. Front. Immunol. 2018. 9. 585. Published 2018, Mar 23. doi: 10.3389/fimmu.2018.00585.
- Lifshitz F., Lifshitz J.Z. Globesity: the root causes of the obesity epidemic in the USA and now worldwide. Pediatr. Endocrinol. Rev. 2014. 12(1). 17-34.
- Lu F.B., Hu E.D., Xu L.M. et al. The relationship between obesity and the severity of non-alcoholic fatty liver disease: systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2018. 12(5). 491-502. doi: 10.1080/17474124.2018.1460202.
- Luckheeram R.V., Zhou R., Verma A.D., Xia B. CD4⁺T cells: differentiation and functions. Clin. Dev. Immunol. 2012. 925135. doi: 10.1155/2012/925135.
- Lui P.P., Cho I., Ali N. Tissue regulatory T cells [published online ahead of print, 2020 May 28]. Immunology. 2020. doi: 10.1111/imm.13208. doi:10.1111/imm.13208.
- Lynch L., Michelet X., Zhang S. et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue. Nat. Immunol. 2015. 16(1). 85-95. doi: 10.1038/ni.3047.
- Ma C.S., Wong N., Rao G. et al. Unique and shared signaling pathways cooperate to regulate the differentiation of human CD4+ T cells into distinct effector subsets. J. Exp. Med. 2016. 213(8). 1589-1608. doi: 10.1084/jem.20151467.
- Matarese G., Procaccini C., De Rosa V., Horvath T.L., La Cava A. Regulatory T cells in obesity: the leptin connection. Trends Mol. Med. 2010. 16(6). 247-256. doi: 10.1016/j.molmed.2010.04.002.
- McDonnell W.J., Koethe J.R., Mallal S.A. et al. High CD8 T-Cell Receptor Clonality and Altered CDR3 Properties Are Associated With Elevated Isolevuglandins in Adipose Tissue During Diet-Induced Obesity. Diabetes. 2018. 67(11). 2361-2376. doi: 10.2337/db18-0040.
- McLaughlin T., Liu L.F., Lamendola C. et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014. 34(12). 2637-2643. doi: 10.1161/ATVBAHA.114.304636.
- Misumi I., Starmer J., Uchimura T., Beck M.A., Magnuson T., Whitmire J.K. Obesity Expands a Distinct Population of T Cells in Adipose Tissue and Increases Vulnerability to Infection. Cell. Rep. 2019. 27(2). 514-524. e5. doi: 10.1016/j.celrep.2019.03.030.
- Miyara M., Yoshioka Y., Kitoh A. et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009. 30(6). 899-911. doi: 10.1016/j.immuni.2009.03.019.
- Molofsky A.B., Van Gool F., Liang H.E. et al. Interleukin-33 and Interferon-γ Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity. 2015. 43(1). 161-174. doi: 10.1016/j.immuni.2015.05.019.
- Morin S.O., Poggi M., Alessi M.C., Landrier J.F., Nunès J.A. Modulation of T Cell Activation in Obesity. Antioxid Redox Signal. 2017. 26(10). 489-500. doi: 10.1089/ars.2016.6746.
- Naylor C., Petri W.A. Jr. Leptin Regulation of Immune Responses. Trends Mol. Med. 2016. 22(2). 88-98. doi: 10.1016/j.molmed.2015.12.001.
- Nishimura S., Manabe I., Nagasaki M. et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009. 15(8). 914-920. doi: 10.1038/nm.1964.
- Oishi Y., Manabe I. Integrated regulation of the cellular metabolism and function of immune cells in adipose tissue. Clin. Exp. Pharmacol. Physiol. 2016. 43(3). 294-303. doi: 10.1111/1440-1681.12539.
- Onishi R.M., Gaffen S.L. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010. 129(3). 311-321. doi: 10.1111/j.1365-2567.2009.03240.x.
- Pacifico L., Di Renzo L., Anania C. et al. Increased T-helper interferon-gamma-secreting cells in obese children. Eur. J. Endocrinol. 2006. 154(5). 691-697. doi: 10.1530/eje.1.02138.
- Pandolfi J.B., Ferraro A.A., Sananez I. et al. ATP-Induced Inflammation Drives Tissue-Resident Th17 Cells in Metabolically Unhealthy Obesity. J. Immunol. 2016. 196(8). 3287-3296. doi: 10.4049/jimmunol.1502506.
- Panduro M., Benoist C., Mathis D. Tissue Tregs. Ann. Rev. Immunol. 2016. 34. 609-633. doi: 10.1146/annurev-immunol-032712-095948.
- Plank M.W., Kaiko G.E., Maltby S. et al. Th22 Cells Form a Distinct Th Lineage from Th17 Cells In Vitro with Unique Transcriptional Properties and Tbet-Dependent Th1 Plasticity. J. Immunol. 2017. 198(5). 2182-2190. doi: 10.4049/jimmunol.1601480.
- Rausch M.E., Weisberg S., Vardhana P., Tortoriello D.V. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. (Lond). 2008. 32(3). 451-463. doi: 10.1038/sj.ijo.0803744.
- Read K.A., Powell M.D., Sreekumar B.K., Oestreich K.J. In Vitro Differentiation of Effector CD4+ T Helper Cell Subsets. Methods Mol. Biol. 2019. 1960. 75-84. doi: 10.1007/978-1-4939-9167-9_6.
- Revelo X.S., Tsai S., Lei H. et al. Perforin is a novel immune regulator of obesity-related insulin resistance. Diabetes. 2015. 64(1). 90-103. doi: 10.2337/db13-1524.
- Rheinheimer J., de Souza B.M., Cardoso N.S., Bauer A.C., Crispim D. Current role of the NLRP3 inflammasome on obesity and insulin resistance: A systematic review. Metabolism. 2017. 74. 1-9. doi: 10.1016/j.metabol.2017.06.002.
- Rocha V.Z., Folco E.J., Sukhova G. et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ. Res. 2008. 103(5). 467-476. doi: 10.1161/CIRCRESAHA.108.177105.
- Ruiz de Morales J.M.G., Puig L., Daudén E. et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun Rev. 2020. 19(1). 102429. doi: 10.1016/j.autrev.2019.102429.
- Sabat R., Wolk K., Loyal L., Döcke W.D., Ghoreschi K. T cell pathology in skin inflammation. Semin Immunopathol. 2019. 41(3). 359-377. doi: 10.1007/s00281-019-00742-7.
- Sabat R., Wolk K. Deciphering the role of interleukin-22 in metabolic alterations. Cell. Biosci. 2015. 5. 68. Published 2015, Dec 15. doi: 10.1186/s13578-015-0060-8.
- Saklayen M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens Rep. 2018. 20(2). 12. Published 2018, Feb 26. doi: 10.1007/s11906-018-0812-z.
- Salastekar N., Desai T., Hauser T. et al. Salsalate improves glycaemia in overweight persons with diabetes risk factors of stable statin-treated cardiovascular disease: A 30-month randomized placebo-controlled trial. Diabetes Obes. Metab. 2017. 19(10). 1458-1462. doi: 10.1111/dom.12940.
- Salomon R.G., Bi W. Isolevuglandin adducts in disease. Antioxid Redox Signal. 2015. 22(18). 1703-1718. doi: 10.1089/ars.2014.6154.
- Samji T., Khanna K.M. Understanding memory CD8+ T cells. Immunol Lett. 2017. 185. 32-39. doi: 10.1016/j.imlet.2017.02.012.
- Sauls R.S., McCausland C., Taylor B.N. Histology, T-Cell Lymphocyte. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2020.
- Schmidleithner L., Thabet Y., Schönfeld E. et al. Enzymatic Activity of HPGD in Treg Cells Suppresses Tconv Cells to Maintain Adipose Tissue Homeostasis and Prevent Metabolic Dysfunction. Immunity. 2019. 50(5). 1232-1248. e14. doi: 10.1016/j.immuni.2019.03.014.
- Shevach E.M. Foxp3+ T Regulatory Cells: Still Many Unanswered Questions-A Perspective After 20 Years of Study. Front. Immunol. 2018. 9. 1048. Published 2018, May 15. doi: 10.3389/fimmu.2018.01048.
- Shevyrev D., Tereshchenko V. Treg Heterogeneity, Function, and Homeostasis. Front. Immunol. 2020. 10. 3100. Published 2020, Jan 14. doi: 10.3389/fimmu.2019.03100.
- Shi H., Chi H. Metabolic Control of Treg Cell Stability, Plasticity, and Tissue-Specific Heterogeneity. Front. Immunol. 2019. 10. 2716. Published 2019, Dec 11. doi: 10.3389/fimmu.2019.02716.
- Sloan-Lancaster J., Abu-Raddad E., Polzer J. et al. Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1β antibody, in patients with type 2 diabetes. Diabetes Care. 2013. 36(8). 2239-2246. doi: 10.2337/dc12-1835.
- Sonnenberg G.F., Fouser L.A., Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 2011. 12(5). 383-390. doi: 10.1038/ni.2025.
- Spallanzani R.G., Zemmour D., Xiao T. et al. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci. Immunol. 2019. 4(35). eaaw3658. doi: 10.1126/sciimmunol.aaw3658.
- Stanley T.L., Zanni M.V., Johnsen S. et al. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J. Clin. Endocrinol. Metab. 2011. 96(1). 146-150. doi: 10.1210/jc.2010-1170.
- Stolarczyk E., Vong C.T., Perucha E. et al. Improved insulin sensitivity despite increased visceral adiposity in mice deficient for the immune cell transcription factor T-bet. Cell. Metab. 2013. 17(4). 520-533. doi: 10.1016/j.cmet.2013.02.019.
- Strissel K.J., DeFuria J., Shaul M.E., Bennett G., Greenberg A.S., Obin M.S. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring). 2010. 18(10). 1918-1925. doi: 10.1038/oby.2010.1.
- Terrell C.E., Jordan M.B. Perforin deficiency impairs a critical immunoregulatory loop involving murine CD8(+) T cells and dendritic cells. Blood. 2013. 121(26). 5184-5191. doi: 10.1182/blood-2013-04-495309.
- Travers R.L., Motta A.C., Betts J.A., Bouloumié A., Thompson D. The impact of adiposity on adipose tissue-resident lymphocyte activation in humans. Int. J. Obes. (Lond.). 2015. 39(5). 762-769. doi: 10.1038/ijo.2014.195.
- Trim W., Turner J.E., Thompson D. Parallels in Immunometabolic Adipose Tissue Dysfunction with Ageing and Obesity. Front. Immunol. 2018. 9. 169. Published 2018, Feb 9. doi: 10.3389/fimmu.2018.00169.
- Umano G.R., Pistone C., Tondina E. et al. Pediatric Obesity and the Immune System. Front. Pediatr. 2019. 7. 487. Published 2019, Nov 22. doi: 10.3389/fped.2019.00487.
- Ussar S., Lee K.Y., Dankel S.N. et al. ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci. Transl. Med. 2014. 6(247). 247ra103. doi: 10.1126/scitranslmed.3008490.
- Van Coillie S., Wiernicki B., Xu J. Molecular and Cellular Functions of CTLA-4. Adv Exp. Med. Biol. 2020. 1248. 7-32. doi: 10.1007/978-981-15-3266-5_2.
- van der Weerd K., Dik W.A., Schrijver B. et al. Morbidly obese human subjects have increased peripheral blood CD4+ T cells with skewing toward a Treg- and Th2-dominated phenotype. Diabetes. 2012. 61(2). 401-408. doi: 10.2337/db11-1065.
- Van Herck M.A., Weyler J., Kwanten W.J. et al. The Differential Roles of T Cells in Non-alcoholic Fatty Liver Disease and Obesity. Front. Immunol. 2019. 10. 82. Published 2019, Feb 6. doi: 10.3389/fimmu.2019.00082.
- Vasanthakumar A., Moro K., Xin A. et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells [published correction appears in Nat. Immunol. 2015 May. 16(5). 544]. Nat. Immunol. 2015. 16(3). 276-285. doi: 10.1038/ni.3085.
- Vorotnikov A.V., Stafeev I.S., Menshikov M.Y., Shestakova M.V., Parfyonova Y.V. Latent Inflammation and Defect in Adipocyte Renewal as a Mechanism of Obesity-Associated Insulin Resistance. Biochemistry (Mosc.). 2019. 84(11). 1329-1345. doi: 10.1134/S0006297919110099.
- Wang Q., Li D., Zhu J. et al. Perforin Acts as an Immune Regulator to Prevent the Progression of NAFLD. Front. Immunol. 2020. 11. 846. Published 2020, May 22. doi: 10.3389/fimmu.2020.00846.
- Wang Q., Wu H. T Cells in Adipose Tissue: Critical Players in Immunometabolism. Front. Immunol. 2018. 9. 2509. Published 2018, Oct 30. doi: 10.3389/fimmu.2018.02509.
- Warbrick I., Rabkin S.W. Hypoxia-inducible factor 1-alpha (HIF-1α) as a factor mediating the relationship between obesity and heart failure with preserved ejection fraction. Obes. Rev. 2019. 20(5). 701-712. doi: 10.1111/obr.12828.
- Weinstock A., Moura Silva H., Moore K.J., Schmidt A.M., Fisher E.A. Leukocyte Heterogeneity in Adipose Tissue, Including in Obesity. Circ. Res. 2020. 126(11). 1590-1612. doi: 10.1161/CIRCRESAHA.120.316203.
- Wiebe N., Stenvinkel P., Tonelli M. Associations of Chronic Inflammation, Insulin Resistance, and Severe Obesity With Mortality, Myocardial Infarction, Cancer, and Chronic Pulmonary Disease. JAMA Netw Open. 2019. 2(8). e1910456. Published 2019, Aug 2. doi: 10.1001/jamanetworkopen.2019.10456.
- Winer S., Chan Y., Paltser G. et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009. 15(8). 921-929. doi: 10.1038/nm.2001.
- Wu D., Molofsky A.B., Liang H.E. et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011. 332(6026). 243-247. doi: 10.1126/science.1201475.
- Wu H., Ballantyne C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020. 126(11). 1549-1564. doi: 10.1161/CIRCRESAHA.119.315896.
- Wu K.K., Cheung S.W., Cheng K.K. NLRP3 Inflammasome Activation in Adipose Tissues and Its Implications on Metabolic Diseases. Int. J. Mol. Sci. 2020. 21(11). 4184. Published 2020, Jun 11. doi: 10.3390/ijms21114184.
- Wu X., Tian J., Wang S. Insight Into Non-Pathogenic Th17 Cells in Autoimmune Diseases. Front. Immunol. 2018. 9. 1112. Published 2018, May 28. doi: 10.3389/fimmu.2018.01112.
- Xiao L., Yang X., Lin Y. et al. Large adipocytes function as antigen-presenting cells to activate CD4(+) T cells via upregulating MHCII in obesity. Int. J. Obes. (Lond.). 2016. 40(1). 112-120. doi: 10.1038/ijo.2015.145.
- Yang H., Youm Y.H., Vandanmagsar B. et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J. Immunol. 2010. 185(3). 1836-1845.
- Yeste A., Mascanfroni I.D., Nadeau M. et al. IL-21 induces IL-22 production in CD4+ T cells. Nat. Commun. 2014. 5. 3753. Published 2014, May 6. doi: 10.1038/ncomms4753.
- Zapata-Gonzalez F., Auguet T., Aragonès G. et al. Interleukin-17A Gene Expression in Morbidly Obese Women. Int. J. Mol. Sci. 2015. 16(8). 17469-17481. Published 2015, Jul 30. doi: 10.3390/ijms160817469.
- Zatterale F., Longo M., Naderi J. et al. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020. 10. 1607. Published 2020, Jan 29. doi: 10.3389/fphys.2019.01607.
- Zeng Q., Sun X., Xiao L., Xie Z., Bettini M., Deng T. A Unique Population: Adipose-Resident Regulatory T Cells. Front. Immunol. 2018. 9. 2075. Published 2018, Sep 28. doi: 10.3389/fimmu.2018.02075.
- Zeyda M., Huber J., Prager G., Stulnig T.M. Inflammation correlates with markers of T-cell subsets including regulatory T cells in adipose tissue from obese patients. Obesity (Silver Spring). 2011. 19(4). 743-748. doi: 10.1038/oby.2010.123.
- Zhao R., Tang D., Yi S. et al. Elevated peripheral frequencies of Th22 cells: a novel potent participant in obesity and type 2 diabetes. PLoS One. 2014. 9(1). e85770. Published 2014, Jan 17. doi: 10.1371/journal.pone.0085770.
- Zhao Y., Lin L., Li J. et al. CD4+ T cells in obesity and obesity-associated diseases. Cell. Immunol. 2018. 332. 1-6. doi: 10.1016/j.cellimm.2018.08.013.
- Zúñiga L.A., Shen W.J., Joyce-Shaikh B. et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity [published correction appears in J Immunol. 2011 Jan 15. 186(2). 1291]. J. Immunol. 2010. 185(11). 6947-6959. doi: 10.4049/jimmunol.1001269.

/86.jpg)
/87.jpg)
/88.jpg)
/90.jpg)
/92.jpg)
/95.jpg)
/97.jpg)
/98.jpg)