Международный эндокринологический журнал Том 16, №8, 2020
Вернуться к номеру
Взаємозв’язок між рівнем тиреотропного гормону, інсулінорезистентністю та серцево-судинними факторами ризику в пацієнтів з ожирінням та субклінічним гіпотиреозом
Авторы: N.V. Pasiechko(1), Yu.V. Yevstratieva(1, 2)
(1) — I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
(2) — Khmelnytskyi City Medical and Diagnostic Centre, Khmelnytskyi, Ukraine
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
Актуальність. Епідемія надмірної ваги та ожиріння розглядається як головний виклик системі охорони здоров’я і потребує проведення профілактики. Взаємозв’язок між функціональним станом щитоподібної залози, масою тіла та гомеостазом жирової тканини перебуває в центрі уваги декількох досліджень протягом останніх років, але причинно-наслідкові зв’язки між цими параметрами чітко не встановлені. Мета: дослідити взаємозв’язок між вмістом тиреотропного гормону (ТТГ), інсулінорезистентністю (ІР) та факторами серцево-судинного ризику у вибірці людей із ожирінням та субклінічним гіпотиреозом. Матеріали та методи. Проведено ретроспективний аналіз 145 осіб з ожирінням. Проаналізовано рівні ТТГ та вільного тироксину (вT4), антропометричні показники та результати лабораторних досліджень. Результати. У 23 осіб рівень ТТГ перевищував нормальний рівень (субклінічний гіпотиреоз). Обвід талії (ОТ) у цих осіб був вірогідно вищим, ніж в осіб у стані еутиреозу. Рівень ТТГ у сироватці крові позитивно корелював з індексом HOMA-IR, вмістом тригліцеридів та ліпопротеїнів високої щільності (ЛПВЩ). З використанням ТТГ та індексу маси тіла як незалежних змінних було показано, що рівні ТТГ незалежно пов’язані з HOMA-IR (p = 0,002) та вмістом тригліцеридів (p = 0,006). Серед еутиреоїдних пацієнтів в осіб зі значеннями ТТГ < 2,5 мМО/мл спостерігалися статистично значуще зниження співвідношення ОТ і обводу стегон, рівня ЛПВЩ та показників HOMA-IR, а також тенденція до зниження значень ОТ. Висновки. Субклінічний гіпотиреоз у людей з ожирінням асоціюється зі збільшенням маси вісцерального жиру. У цій вибірці людей з ожирінням рівні ТТГ вірогідно пов’язані з наявністю інсулінорезистентності.
Актуальность. Эпидемия избыточного веса и ожирения представляет собой серьезную проблему для системы здравоохранения во всем мире и требует проведения профилактических мероприятий. Предполагаемые взаимосвязи между гормонами щитовидной железы, массой тела и гомеостазом жировой ткани находятся в центре внимания нескольких исследований в последние годы, однако причинно-следственные связи между этими параметрами четко не установлены. Цель: изучить взаимосвязь между уровнем тиреотропного гормона (ТТГ), инсулинорезистентностью (ИР) и факторами риска сердечно-сосудистых заболеваний в выборке лиц с ожирением и субклиническим гипотиреозом. Материалы и методы. Проведен ретроспективный продольный анализ 145 лиц с ожирением. Проанализированы уровни ТТГ и свободного тироксина (свT4), антропометрические измерения и результаты лабораторных тестов. Результаты. У 23 человек уровень ТТГ был выше нормы (субклинический гипотиреоз). Окружность талии (ОТ) у них была достоверно выше, чем у людей в состоянии эутиреоза. Уровень ТТГ в сыворотке положительно коррелировал с индексом инсулинорезистентности (HOMA-IR), содержанием триглицеридов и холестерина липопротеинов высокой плотности (ЛПВП). С использованием ТТГ и индекса массы тела в качестве независимых переменных было показано, что уровни ТТГ независимо связаны с HOMA-IR (p = 0,002) и содержанием триглицеридов (p = 0,006). Среди эутиреоидных пациентов лица со значениями ТТГ < 2,5 мМЕ/мл демонстрировали статистически значимое снижение соотношения ОТ и охвата бедер, уровня ЛПВП и показателей HOMA-IR, а также тенденцию к более низким значениям ОТ. Выводы. Субклинический гипотиреоз у людей с ожирением связан с избыточным наличием висцеральной жировой ткани. В настоящей выборке людей с ожирением уровни ТТГ связаны с инсулинорезистентностью.
Background. The epidemic of overweight and obesity presents a major challenge to chronic disease prevention and health across the life course around the world. The putative relationships between thyroid hormones, body weight, and adipose tissue homeostasis have been the focus of several studies in recent years, but the causal relationships between these parameters have not been well established. The purpose of the study: to investigate the relationship between serum thyroid-stimulating hormone (TSH), insulin resistance (IR), and cardiovascular risk factors in a sample of obese people with subclinical hypothyroidism. Materials and methods. A retrospective, longitudinal analysis of 145 obese patients was performed. The TSH and free thyroxine (fT4) levels, anthropometric measurements, and laboratory test results were analyzed. Results. Twenty-three individuals presented with TSH levels above the normal level (subclinical hypothyroidism). Their waist circumference (WC) was significantly higher than that of euthyroid individuals. Serum TSH positively correlated with the homeostasis model assessment of insulin resistance (HOMA-IR) index, triglycerides, and high-density lipoprotein cholesterol (HDL-C). Using TSH and body mass index as independent variables, TSH levels were shown to be independently related to HOMA-IR (p = 0.002) and triglycerides (p = 0.006). Among euthyroid subjects, individuals with TSH values < 2.5 mIU/ml exhibited statistically significant decreases in waist-to-hip ratio, HDL-C levels, and HOMA-IR scores and a tendency toward lower WC values. Conclusions. Subclinical hypothyroidism in overweight and obese people appears to be associated with excess weight, especially visceral weight. In the present sample of obese patients, TSH levels appear to be associated with insulin resistance.
субклінічний гіпотиреоз; ожиріння; серцево-судинні фактори ризику
субклинический гипотиреоз; ожирение; факторы риска сердечно-сосудистых заболеваний
subclinical hypothyroidism; obesity; cardiovascular risk factors
Introduction
Materials and methods
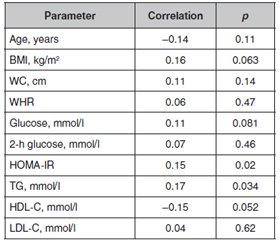
Results
/42_2.jpg)
Discussion
Conclusions
- Hruby A., Hu F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics. 2015. 33 (7). 673-89. doi: 10.1007/s40273-014-0243-x.
- Dyachuk D., Yaschenko Y., Zabolotna I., Yaschenko L. Prevalence of excessive body weight and obesity among children; organization of prevention of child obesity in Ukraine. Georgian Med. News. 2019. 289. 62-67. PMID: 31215881.
- Yakovenko V., Henn L., Bettendorf M., Zelinska N., Soloviova G., Hoffmann G.F., Grulich-Henn J. Risk Factors for Childhood Overweight and Obesity in Ukraine and Germany. J. Clin. Res. Pediatr. Endocrinol. 2019. 11 (3). 247-252. doi: 10.4274/jcrpe.galenos.2019.2018.0157.
- Han T.S., Lean M.E. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 2016. 5. doi: 10.1177/2048004016633371.
- Laurberg P., Knudsen N., Andersen S., Carlé A., Pedersen I.B., Karmisholt J. Thyroid function and obesity. Eur. Thyroid J. 2012. 1 (3). 159-67. doi: 10.1159/000342994.
- Sanyal D., Raychaudhuri M. Hypothyroidism and obesity: An intriguing link. Indian J. Endocrinol. Metab. 2016. 20 (4). 554-7. doi: 10.4103/2230-8210.183454.
- Gajda S.N., Kuryłowicz A., Żach M., Bednarczuk T., Wyleżoł M. Diagnosis and treatment of thyroid disorders in obese patients — what do we know? Endokrynol. Pol. 2019. 70 (3). 271-276. doi: 10.5603/EP.a2018.0089.
- Nyrnes A., Jorde R., Sundsfjord J. Serum TSH is positively associated with BMI. Int. J. Obes. (Lond). 2006. 30 (1). 100-5. doi: 10.1038/sj.ijo.0803112.
- Martin S.S., Daya N., Lutsey P.L., Matsushita K., Fretz A., McEvoy J.W., Blumenthal R.S. et al. Thyroid Function, Cardiovascular Risk Factors, and Incident Atherosclerotic Cardiovascular Disease: The Atherosclerosis Risk in Communities (ARIC) Study. J. Clin. Endocrinol. Metab. 2017. 102 (9). 3306-3315. doi: 10.1210/jc.2017-00986.
- Ichiki T. Thyroid hormone and atherosclerosis. Vascul. Pharmacol. 2010. 52 (3–4). 151-6. doi: 10.1016/j.vph.2009.09.004.
- Kahaly G.J. Cardiovascular and atherogenic aspects of subclinical hypothyroidism. Thyroid. 2000. 10 (8). 665-79. doi: 10.1089/10507250050137743.
- Rodondi N., den Elzen W.P., Bauer D.C., Cappola A.R., Razvi S., Walsh J.P., Asvold B.O. et al. Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010. 304 (12). 1365-74. doi: 10.1001/jama.2010.1361.
- Berta E., Lengyel I., Halmi S., Zrínyi M., Erdei A., Harangi M., Páll D., Nagy E.V., Bodor M. Hypertension in Thyroid Disorders. Front. Endocrinol. (Lausanne). 2019. 10. 482. doi: 10.3389/fendo.2019.00482.
- Young W.F., Calhoun D.A., Lenders J.W.M., Stowasser M., Textor S.C. Screening for endocrine hypertension: an endocrine society scientific statement. Endocr. Rev. 2017. 38. 103-22. doi: 10.1210/er.2017-00054.
- Yin D.T., He H., Yu K., Xie J., Lei M., Ma R. et al. The association between thyroid cancer and insulin resistance, metabolic syndrome and its components: a systematic review and meta-analysis. Int. J. Surg. 2018. 57. 66-75. doi: 10.1016/j.ijsu.2018.07.013.
- Garber J.R., Cobin R.H., Gharib H., Hennessey J.V., Klein I., Mechanick J.I. et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012. 22. 1200-35. doi: 10.1089/thy.2012.0205.
- Biondi B., Cappola A.R., Cooper D.S. Subclinical Hypothyroidism: A Review. JAMA. 2019. 322 (2). 153-160. doi: 10.1001/jama.2019.9052.
- Deshmukh V., Behl A., Iyer V., Joshi H., Dholye J.P., Varthakavi P.K. Prevalence, clinical and biochemical profile of subclinical hypothyroidism in normal population in Mumbai. Indian J. Endocrinol. Metab. 2013. 17 (3). 454-9. doi: 10.4103/2230-8210.111641.
- Janssen I.M., Homan J., Schijns W., Betzel B., Aarts E.O., Berends F.J., de Boer H. Subclinical hypothyroidism and its relation to obesity in patients before and after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2015. 11 (6). 1257-63. doi: 10.1016/j.soard.2015.02.021.
- Pankiv V.I., Yuzvenko T.Yu., Pankiv I.V. Type 2 diabetes mellitus and subclinical hypothyroidism: focusing on the role of cholecalciferol. Problems of Endocrine Pathology. 2019. 2. 46-51. doi: 10.21856/j-PEP.2019.2.07.
- Jin H.Y. Prevalence of subclinical hypothyroidism in obese children or adolescents and association between thyroid hormone and the components of metabolic syndrome. J. Paediatr. Child Health. 2018. 54 (9). 975-980. doi: 10.1111/jpc.13926.
- Sanyal D., Raychaudhuri M. Hypothyroidism and obesity: An intriguing link. Indian J. Endocrinol. Metab. 2016. 20 (4). 554-7. doi: 10.4103/2230-8210.183454.

/41.jpg)
/42.jpg)