Журнал "Гастроэнтерология" Том 54, №2, 2020
Вернуться к номеру
Intensity of systemic proteolysis and endotoxicosis in patients with non-alcoholic steatohepatitis associated with obesity and comorbid chronic obstructive pulmonary disease in the dynamics of treatment with hepatoprotectors
Авторы: O.S. Khukhlina, O.Ye. Hrinyuk, O.D. Liakhovych
Bukovinian State Medical University, Chernivtsi, Ukraine
Рубрики: Гастроэнтерология
Разделы: Клинические исследования
Версия для печати
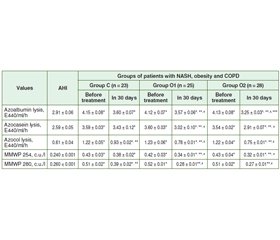
Актуальність. Поширеність захворюваності на неалкогольний стеатогепатит (НАСГ) та хронічне обструктивне захворювання легень (ХОЗЛ) набуває глобального значення серед населення економічно розвинених країн світу з тенденцією зростання і в Україні. Мета дослідження: визначення інтенсивності системного протеолізу та ендогенної інтоксикації до лікування та в динаміці застосування гепатопротекторів у хворих на НАСГ на тлі ожиріння та коморбідного ХОЗЛ. Матеріали та методи. 76 хворих на НАСГ, ожиріння І ст. та ХОЗЛ 2–3 D пройшли обстеження та були розподілені на 3 групи залежно від призначеного лікування. До першої групи — контрольної (К) — увійшли 23 хворі, якi отримували базисну терапію НАСГ (комплекс есенціальних фосфоліпідів 300 мг по 2 капсули 3 рази на день) упродовж 30 днів та базисну терапію ХОЗЛ. 25 хворих (група 2 — основна, О1), крім аналогічної терапії ХОЗЛ, для лікування НАСГ замість комплексу есенціальних фосфоліпідів отримували антраль 200 мг 3 рази на день упродовж 30 днів. Третя група (основна, О2) — 28 хворих, крім аналогічної терапії ХОЗЛ, для лікування НАСГ замість комплексу есенціальних фосфоліпідів отримували антраль 200 мг 3 рази на день та додатково полікозанол 20 мг після вечері впродовж 30 днів. Групу порівняння становили 30 практично здорових осіб. Результати. Запропонована терапія антралем вплинула на зменшення інтенсивності лізису азоальбуміну, азоказеїну та азоколу у хворих групи О2: на 30 день зазначений показник знизився відповідно в 1,3; 1,2 та 1,6 раза (р < 0,05), у хворих групи О1: на 30-й день — в 1,2; 1,2 та 1,6 раза відповідно (р < 0,05) порівняно з величинами до лікування. У групі К зниження відбувалося менш інтенсивно (р < 0,05): вірогідно змінювався лише показник лізису азоколу — знизився в 1,3 раза (р < 0.05) за наявності вірогідної різниці з показниками у групах О1 та О2 (р < 0,05). Висновки. Призначення антралю упродовж 30 днів призвело до істотної корекції протеїназо-інгібіторного гомеостазу у хворих на НАСГ із ожирінням та ХОЗЛ, що супроводжувалось вірогідним зниженням ендотоксикозу (р < 0,05) та ушкоджувальної дії системного протеолізу (р < 0,05).
Актуальность. Распространенность заболеваемости неалкогольным стеатогепатитом (НАСГ) и хронической обструктивной болезнью легких (ХОБЛ) приобретает глобальное значение среди населения экономически развитых стран мира с тенденцией роста и в Украине. Цель исследования: определение интенсивности системного протеолиза и эндогенной интоксикации до лечения и в динамике применения антраля у больных НАСГ на фоне ожирения и коморбидной ХОБЛ. Материалы и методы. 76 больных НАСГ на фоне ожирения I ст. и ХОБЛ 2–3 D прошли обследование и были распределены на 3 группы в зависимости от назначенного лечения. В первую группу — контрольную (К) — вошли 23 больных, получавших базисную терапию НАСГ (комплекс эссенциальных фосфолипидов 300 мг по 2 капсулы 3 раза в день) на протяжении 30 дней и базисную терапию ХОБЛ. 25 больных (группа 2 — основная, О1), кроме аналогичной терапии ХОБЛ, для лечения НАСГ вместо комплекса эссенциальных фосфолипидов получали антраль 200 мг 3 раза в день на протяжении 30 дней. Третья группа (основная, О2) — 28 больных, кроме аналогичной терапии ХОБЛ, для лечения НАСГ вместо комплекса эссенциальных фосфолипидов получали антраль 200 мг 3 раза в день и дополнительно поликозанол 20 мг после ужина в течение 30 дней. Группу сравнения составили 30 практически здоровых лиц. Результаты. Предложенная терапия антралем повлияла на уменьшение интенсивности лизиса азоальбумина, азоказеина и азокола у больных группы О2: на 30 день указанный показатель снизился соответственно в 1,3; 1,2 и 1,6 раза (р < 0,05), у больных группы О1: на 30 день — соответственно в 1,2; 1,2 и 1,6 раза (р < 0,05) по сравнению с данными до лечения. В группе К снижение происходило менее интенсивно (р < 0,05): достоверно менялся только показатель азокола — снизился в 1,3 раза (р < 0,05) при наличии достоверной разницы с показателями в группах О1 и О2 (р < 0,05). Выводы. Назначение антраля на фоне базисной терапии в течение 30 дней привело к существенной коррекции протеиназo-ингибиторного гомеостаза у больных НАСГ с ожирением и ХОБЛ, сопровождалось достоверным снижением эндотоксикоза (р < 0,05) и повреждающего действия системного протеолиза (р < 0,05).
Background. The prevalence of non-alcoholic steatohepatitis (NASH) and chronic obstructive pulmonary disease (COPD) is gaining global significance in the population of economically developed countries with a growing trend in Ukraine. The purpose was the determination of the intensity of systemic proteolysis and endogenous intoxication before treatment and the efficacy of hepatoprotective therapy in patients with NASH against the background of obesity, depending on the comorbidity of COPD. Materials and methods. Seventy-six patients with NASH, grade 1 obesity and COPD 2–3 D were screened and divided into 3 groups according to the treatment received. The control group (C group) consisted of 23 patients receiving basic treatment for NASH (essential fatty acids complex 300 mg 2 capsules 3 times daily) for 30 days and baseline COPD therapy. Twenty-five patients (group 2 — primary, O1), in addition to similar COPD therapy, for the treatment of NASH, instead of essential fatty acids complex, received antral at a dose of 200 mg 3 times a day for 30 days. The third group (basic, O2) involving 28 patients with NASH, grade 1 obesity and COPD 2–3 D, in addition to similar COPD therapy, for the treatment of NASH, instead of essential fatty acids complex, received antral at a dose of 200 mg 3 times daily and, additionally, policosanol at a dose of 20 mg after the dinner for 30 days. The comparison group consisted of 30 apparently healthy individuals. Results. The proposed therapy with antral reduced the intensity of lysis of azoalbumin, azocasein and azocol in patients of group O2: at day 30, the decrease was 1.3, 1.2 and 1.6 times (p < 0.05), respectively, in patients of the group O1: on day 30, the decrease was 1.2, 1.2 and 1.6 times (p < 0.05), respectively, compared to the pre-treatment values. In the group C, the values decreased less intensively (p < 0.05): only the azocol values were likely to change — 1.3 times (p < 0.05) with the presence of a significant difference with the groups O1 and O2 (p < 0.05). Conclusions. The combined administration of antral for 30 days resulted in a significant correction of proteinase-inhibitory homeostasis in patients with NASH associated with obesity and COPD, which was accompanied by a significant decrease in endotoxicosis (p < 0.05) and damaging effect of systemic proteolysis (p < 0.05).
неалкогольний стеатогепатит; хронічне обструктивне захворювання легень; протеоліз; ендотоксикоз
неалкогольный стеатогепатит; хроническая обструктивная болезнь легких; протеолиз; эндотоксикоз
non-alcoholic steatohepatitis; chronic obstructive pulmonary disease; proteolysis; endotoxicosis
Introduction
Material and methods
Results
Discussion
Conclusions
- Анохіна А.А. Неалкогольна жирова хвороба печінки як мультисистемне метаболічне захворювання: особливості профілактики та лікування. Гепатологія. 2018. Т. 7, № 3. С. 35-40.
- Хухліна О.С., Антонів А.А., Мандрик О.Є., Гринюк О.Є. Неалкогольна жирова хвороба печінки та коморбідні стани: особливості патогенезу, клініки, діагностики, лікування. Чернівці, 2018. С. 58-61.
- Кобиляк Н.М., Динник О.Б., Кирієнко Д. Сучасні підходи до діагностики та скринінгу метаболічних порушень у хворих із неалкогольною жировою хворобою печінки. Міжнародний ендокринологічний журнал. 2015. Т. 5, № 89. С. 89-99. doi: 10.22141/2224-072.
- Younossi Z.M. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016. Vol. 64. P. 1577-1586. doi: 10.1002/hep.28785.
- Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanya A.J. Mechanisms of NAFLD development and therapeutic strategy. Nature Medicine. 2018 Jul. 24(7). 908-922. doi: 10.1038/s41591-018-0104-9.
- Rinella M.E. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015. P. 313. doi: 10.10012263-73./jama.2015. 5370.
- European Association for the Study of the Liver et al. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Obesity facts. 2016. Vol. 9 (2). P. 65-90. doi: 10.1159/000443344.
- Armstrong M.J., Hull D., Guo K. et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J. Hepatol. 2016. Vol. 64. P. 399-408. PMID: 26394161. doi: 10.1016/j.jhep.2015.08.038.
- Joy T.R., McKenzie C.A., Tirona R.G. et al. Sitagliptin in patients with non-alcoholic steatohepatitis: A randomized, placebo-controlled trial. World J. Gastroenterol. 2017. Vol. 23. P. 141-150. PMID: 28104990. doi: 10.3748/wjg.v23.i1.141.
- Singh S., Khera R., Allen A.M. et al. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: A systematic review and network meta-analysis. Hepatology. 2015. Vol. 62. P. 1417-1432. PMID: 26189925. doi: 10.1002/hep.27999.
- Садикова С.И., Тагаева М.Х., Джалилова С.Х., Рустамова М.Т. Хронические заболевания печени вирусной этиологии — современные принципы терапии препаратом Антраль. Илмий-Амалий Тиббиёт Журнали. 2015. № 2. С. 85-88.
- Кузьмінов Б.П., Матисік С.І., Зазуляк Т.С., Микитчак Т.І. Оцінка гострої токсичності гепатопротектора антралю на альтернативних тест-системах. Environment & Health. 2016. № 2. С. 43-46.
- Дроговоз С.М., Міщенко О.Я., Калько К.О., Богдан Н.С., Геруш О.В. Циркадіанні ритми розвитку експериментального парацетамолового гепатиту та впливу гепатопротекторів на активність прооксидантно/антиоксидантних і цитолітичних процесів. Клінічна фармація. 2019. Т. 23, № 2. С. 15-24. doi: 10.24959/cphj.19.1485.
- Marinangeli C.P.F., Jones P.J.H., Kassis A.N., Eskin M.N.A. Policosanols as Nutraceuticals: Fact or Fiction. Crit. Rev. Food Sci. Nutr. 2010. Vol. 50 (3). P. 259-267. doi: 10.1080/ 10408391003626249.
- Chalasani N., Younossi Z., Lavine J.E. et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018. Vol. 67. P. 28-357. doi: 10.1002/hep.29367.
- Labenz C., Huber Y., Kalliga E. et al. Predictors of advanced fibrosis in non-cirrhotic non-alcoholic fatty liver disease in Germany. Aliment. Pharmacol. Ther. 2018. Vol. 48 (10). P. 1109-1116. doi: 10.1111/apt.14976.
- Kolishetska M.A., Vesklyarova U.P., Zastryzhna M.L. Asthma: shift some indicators proteinase-inhibitory system in the lungs of guinea pigs and their correction of thiotriazolin. Journal of Education, Health and Sport. 2017. Vol. 7 (2). P. 328-336. doi: 10.5281/zenodo.377037.
- Mantovani A., Byrne C.D., Bonora E. et al. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care. 2018 Feb. Vol. 41 (2). P. 372-382. doi: 10.2337/dc17-1902.
- Кryskiv O.I., Kuziv P.P., Babinets L.S. Протеоліз в умовах традиційного лікування і розвантажувально-дієтичної терапії. Здобутки клінічної і експериментальної медицини. 2018. № 4. doi: 10.11603/1811-2471.2017.v0.i4.8387.
- Pang J., Xu W., Zhang X. et al. Significant positive association of endotoxemia with histological severity in 237 patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2017. Vol. 46 (2). P. 175-182. doi: 10.1111/apt.14119.
- Viglino D., Jullian-Desayes I., Minoves M. et al. Nonalcoholic fatty liver disease in chronic obstructive pulmonary disease. Eur. Respir. J. 2017. Vol. 49 (6). doi: 10.1183/13993003.01923-2016.
- Singh D., Agusti A., Anzueto A. et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2019 report. European Respiratory Journal. 2019 May. Vol. 53 (5). 1900164. doi: 10.1183/13993003.00164-2019.
- Анохіна Г.А., Харченко В.Д., Динник О.Б. Роль запалення та метаболічних порушень у прогресуванні хронічних захворювань печінки: профілактика та лікування. Гепатологія. 2018. Т. 15–16, № 436–437. С. 60-62.
- Ривак Т.Б., Коваль А.Я. Вивчення думки населення щодо самолікування гепатотропними лікарськими засобами. Гепатологія. 2019. № 1. С. 35-46.

/38.jpg)