Журнал "Гастроэнтерология" Том 51, №3, 2017
Вернуться к номеру
FibroScan і неінвазивні індекси в діагностиці неалкогольної жирової хвороби печінки
Авторы: Yu.M. Stepanov(1), N.V. Nedzvetskaya(1), V.B. Yagmur(1), D.V. Popok(1), L.M. Shendrik(2)
(1) — State Institution “Institute of Gastroenterology of the National Academy of Medical Sciences of Ukraine”, Dnipro, Ukraine
(2) — State Institution “Dnipropetrovsk Medical Academy of Ministry of Health of Ukraine”, Dnipro, Ukraine
Рубрики: Гастроэнтерология
Разделы: Клинические исследования
Версия для печати
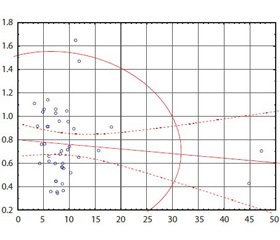
Актуальність. Неалкогольна жирова хвороба печінки (НЖХП) є самостійною нозологічною одиницею, характеризується накопиченням жиру в гепатоцитах, не пов’язаним зі зловживанням алкоголем, і включає широкий спектр порушень — від жирової дистрофії печінки, неалкогольного стеатогепатиту до фіброзу з можливим переходом у цироз печінки. З огляду на поширеність цієї патології, погіршення якості життя хворих, збільшення смертності від ускладнень зростає інтерес до розробки методів для точної й своєчасної оцінки фіброзу. Мета: порівняльна характеристика результатів транзієнтної еластометрії (FibroScan) і неінвазивних лабораторних індексів у визначенні фіброзної трансформації печінки у хворих із неалкогольною жировою хворобою печінки. Матеріали та методи. У дослідження включені пацієнти з НЖХП, які проходили обстеження й лікування у відділенні захворювань печінки та підшлункової залози ДУ «Інститут гастроентерології НАМН України». Обстежені 42 пацієнти з НЖХП, серед яких 18 (45 %) чоловіків і 24 (55 %) жінки. Усім пацієнтам було виконано розрахунок неінвазивних маркерів фіброзу печінки: APRI, FIB-4, співвідношення аланінамінотрансферази/аспартатамінотрансферази, проведено вимірювання жорсткості печінки за допомогою апарату FibroScan. Результати нашої роботи узгоджуються з більшістю досліджень, згідно з якими найбільш ефективним з малоінвазивних індексів є APRI. Висновки. Поєднання транзієнтної еластометрії (FibroScan) з індексом APRI може забезпечити більш ефективний підхід до діагностики фіброзу печінки у хворих з НЖХП.
Актуальность. Неалкогольная жировая болезнь печени (НЖБП) является самостоятельной нозологической единицей, характеризуется накоплением жира в гепатоцитах, не связанным со злоупотреблением алкоголем, и включает широкий спектр нарушений — от жировой дистрофии печени, неалкогольного стеатогепатита до фиброза с возможным исходом в цирроз печени. С учетом распространенности этой патологии, ухудшения качества жизни больных, увеличения смертности от осложнений растет интерес к разработке методов для точной и своевременной оценки фиброза. Цель: сравнительная характеристика результатов транзиентной эластометрии (FibroScan) и неинвазивных лабораторных индексов в определении фиброзной трансформации печени у больных с НЖБП. Материалы и методы. В исследование включены пациенты с НЖБП, которые проходили обследование и лечение в отделении заболеваний печени и поджелудочной железы ГУ «Институт гастроэнтерологии НАМН Украины». Обследовано 42 пациента с НЖБП, среди которых 18 (45 %) мужчин и 24 (55 %) женщины. Всем пациентам был выполнен расчет неинвазивных маркеров фиброза печени: APRI, FIB-4, соотношение аланинаминотрансферазы/аспартатаминотрансферазы, проведено измерение жесткости печени при помощи аппарата FibroScan. Результаты нашей работы согласуются с большинством исследований, согласно которым наиболее эффективным из малоинвазивных индексов является APRI. Выводы. Сочетание транзиентной эластометрии (FibroScan) с индексом APRI может обеспечить более эффективный подход в диагностике фиброза печени у больных с НЖБП.
Background. Non-alcoholic fatty liver disease (NAFLD), an independent nosological entity, is characterized by fat accumulation in hepatocytes not associated with alcohol abuse, and includes a wide spectrum of disorders: from fatty liver, non-alcoholic steatohepatitis to fibrosis with possible outcome in liver cirrhosis. Given the prevalence of this disease, the deterioration of the quality of life of patients, increased mortality from complications, there is a growing interest in developing techniques for accurate and timely assessment of fibrosis. Objective: comparative characteristics of the results of transient elastometry (FibroScan) and non-invasive laboratory indices in the determination of fibrotic transformation of the liver in patients with non-alcoholic fatty liver disease. Materials and methods. The study included patients with NAFLD, who underwent diagnostics and treatment in the department of liver and pancreas of the SI “Institute of Gastroenterology of the NAMS of Ukraine”. Results. We have examined 42 patients with NAFLD, among which 18 (45 %) men and 24 (55 %) women. All patients underwent calculation of non-invasive markers of liver fibrosis: aspartate aminotransferase to platelet ratio index (APRI), fibrosis-4 index, aspartate aminotransferase/alanine aminotransferase ratio, the measurement of liver stiffness using the FibroScan apparatus. Conclusions. Our results are consistent with most studies indicating that the most effective non-invasive index is APRI. The combination of transient elastography (FibroScan) and the APRI index may provide a more effective approach to the diagnosis of liver fibrosis in patients with NAFLD.
неалкогольна жирова хвороба печінки; фіброз печінки; неінвазивні методи діагностики; транзієнтна еластометрія
неалкогольная жировая болезнь печени; фиброз печени; неинвазивные методы диагностики; транзиентная эластография
non-alcoholic fatty liver disease; liver fibrosis; non-invasive diagnostic methods; transient elastography
Introduction
Materials and methods
Results
Discussion
Conclusions
- European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016 Jun;64(6):1388-402. doi: 10.1016/j.jhep.2015.11.004.
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012 Jun;55(6):2005-23. doi: 10.1002/hep.25762.
- Soderberg C, Stal P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010 Feb;51(2):595-602. doi: 10.1002/hep.23314.
- Li H, Wang YJ, Tan K, et al. Prevalence and risk factors of fatty liver disease in Chengdu, Southwest China. Hepatobiliary. Hepatobiliary Pancreat Dis Int. 2009 Aug;8(4):377-82. PMID: 19666406.
- Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol. 2013 May;58(5):1007-19. doi: 10.1016/j.jhep.2012.11.021.
- Friedrich-Rust M, Romen D, Vermehren J, et al. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol. 2012 Mar;81(3):e325-31. doi: 10.1016/j.ejrad.2011.10.029.
- Ying HY, Lu LG, Jing DD, Ni XS. Accuracy of transient elastography in the assessment of chronic hepatitis C-related liver cirrhosis. Clin Invest Med. 2016 Oct 14;39(5):E150-E160. PMID: 27805898.
- Calès P, Boursier J, Lebigot J, et al. Liver fibrosis diagnosis by blood test and elastography in chronic hepatitis C: agreement or combination? Aliment Pharmacol Ther. 2017 Apr;45(7):991-1003. doi: 10.1111/apt.13954.
- Fitzpatrick E, Dhawan A. Noninvasive biomarkers in non-alcoholic fatty liver disease: Current status and a glimpse of the future. World J Gastroenterol. 2014 Aug 21;20(31):10851-63. doi: 10.3748/wjg.v20.i31.10851.
- Kruger FC, Daniels CR, Kidd M, et al. APRI: a simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S Afr Med J. 2011 Jun 27;101(7):477-80. PMID: 21920102.
- Shah AG, Lydecker A, Murray K, et al. Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009 Oct;7(10):1104-12. doi: 10.1016/j.cgh.2009.05.033.
- McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010 Sep;59(9):1265-9. doi: 10.1136/gut.2010.216077.
- Calès P, Lainé F, Boursier J, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol. 2009 Jan;50(1):165-73. doi: 10.1016/j.jhep.2008.07.035.

/28-1.jpg )
/29-1.jpg )
/29-2.jpg )