Резюме
Актуальність. Чітка програма інсулінотерапії є запорукою ефективного лікування пацієнта в стані діабетичного кетоацидозу (ДКА). Підходи до лікування змінювались разом із накопиченням досвіду, проте і зараз думки фахівців стосовно дози та методики проведення інсулінотерапії неоднозначні. Мета дослідження: оцінка ефективності використання інсулінотерапії шляхом постійної інфузії шприцевим насосом в стартовій дозі 0,1 ОД/кг/год під контролем глікемії без використання внутрішньовенної болюсної дози інсуліну та визначення необхідної добової дози інсуліну в першу добу лікування ДКА. Матеріали та методи. Обстежені 55 хворих із ДКА віком від 9 до 65 років. Середній вік хворих становив 31,58 ± 17,18 року, частка пацієнтів жіночої статі серед досліджуваних становила 56 %, чоловічої — 44 %. Пацієнти з цукровим діабетом 1-го типу становили 80 %. Визначення рівня глюкози проводилось у свіжій капілярній крові автоматичним аналізатором глюкози, всім контролювали глікемію через годину після введення 0,1 ОД/кг/год інсуліну та в подальшому проводили корекцію дози за потребою під контролем рівня глюкози. При лікуванні використовували інсуліни короткої дії та аналоги інсулінів ультракороткої дії, підраховували їх сумарну дозу за першу добу лікування. Результати. Рівень глікемії при госпіталізації перебував в межах від 6,7 до 62,2 ммоль/л, у середньому — 23,37 ± 10,16 ммоль/л. Серед досліджуваних глікемія до 13 ммоль/л траплялася у 13 % хворих. Глікемія від 13 до 20 ммоль/л була у 29 % хворих, а гіперглікемія понад 20 ммоль/л — у 58 %. Динаміка рівня глікемії через годину після введення вирахуваної дози інсуліну у 63,6 % хворих була бажаною, зниження глікемії за годину становило 0,5–5,3 ммоль/л, проте відзначалася індивідуальна чутливість до введеної дози інсуліну. Добова доза інсуліну становила від 0,5 до 5,83 ОД/кг. Медіана добової дози інсуліну серед досліджуваних становила 1,36 ОД/кг, найчастіше — 0,92–1,88 ОД/кг за добу [25 %; 75 %]. Висновки. Доза інсуліну в розрахунку 0,1 ОД/кг/год є дієвою, проте через встановлену індивідуальну чутливість до інсуліну вагомим чинником ефективності лікування є контроль глікемії щогодини для своєчасної корекції дози інсуліну.
Актуальность. Четкая программа инсулинотерапии является залогом эффективного лечения пациента в состоянии диабетического кетоацидоза (ДКА). Подходы к лечению менялись вместе с накоплением опыта, но и сейчас мнения специалистов касательно дозы и методики проведения инсулинотерапии неоднозначны. Цель исследования: оценка эффективности методики проведения инсулинотерапии ДКА шприцевым насосом путем постоянной инфузии в стартовой дозе 0,1 ЕД/кг/ч под контролем гликемии без использования болюсной дозы инсулина и определение необходимой суточной дозы инсулина в первые сутки лечения. Материалы и методы. Обследованы 55 больных с ДКА в возрасте от 9 до 65 лет. Средний возраст больных составил 31,58 ± 17,18 года, удельный вес пациентов женского пола среди исследуемых составил 56 %, мужского — 44 %. Пациенты с сахарным диабетом 1-го типа составили 80 % всех исследуемых. Определение уровня глюкозы проводили в свежей капиллярной крови автоматическим анализатором, всем контролировали гликемию после введения 0,1 ЕД/кг/ч инсулина и в дальнейшем проводили коррекцию дозы по необходимости под контролем уровня глюкозы. При лечении использовали инсулины короткого действия и аналоги инсулинов ультракороткого действия, подсчитывали суммарную дозу инсулина за первые сутки лечения. Результаты. Уровень гликемии при поступлении колебался от 6,7 до 62,2 ммоль/л, в среднем — 23,37 ± 10,16 ммоль/л. Среди исследуемых гликемия до 13 ммоль/л встречалась у 13 % больных, гликемия 13–20 ммоль/л — у 29 %, а гликемия свыше 20 ммоль/л — у 58 %. Динамика уровня гликемии после введения рассчитанной дозы инсулина в 63,6 % случаев была желаемой, снижение гликемии за час составило 0,5–5,3 ммоль/л, однако отмечена индивидуальная чувствительность к введенной дозе. Суточная доза инсулина колебалась от 0,5 до 5,83 ЕД/кг. Медиана суточной дозы составляла 1,36 ЕД/кг, чаще — 0,92–1,88 ЕД/кг [25 %; 75 %]. Выводы. Рекомендуемая доза в расчете 0,1 ЕД/кг/ч является действенной, однако из-за выявленной индивидуальной чувствительности к введенному инсулину значимым критерием эффективности лечения становится ежечасный контроль гликемии для своевременной коррекции дозы инсулина.
Background. The well-defined program of the insulin therapy is a guarantee of efficient treatment of a patient with the diabetic ketoacidosis. The approaches to the treatment varied depending on the gained experience, however, the experts’ opinions about the dose and methods of the insulin therapy are still ambiguous. The evaluation of the insulin therapy efficacy via continuous infusion by means of a syringe pump with the initial dose 0.1 unit/kg/h under the control of the glycemia without the usage of a bolus insulin dose IV and the determination of the necessary daily insulin dose on the first day of the diabetic ketoacidosis treatment. The purpose of the study — the evaluation of the insulin therapy efficacy for the diabetic ketoacidosis with syringe pump by means of the continuous infusion with the initial dose 0.1 unit/kg/h under the control of the glycemia as the principal component of the pathogenetic treatment and the determination of the necessary daily insulin dose on the first day of the diabetic ketoacidosis treatment in parallel with the rehydration start by means of physiological solution. Materials and methods. 55 patients with diabetic ketoacidosis aged from 9 to 65 years were examined. The average age of the patients was 31.58 ± 17.18 years. The specific weight of the female patients was 56 %, and male patients — 44 %. 80 % of patients had the type 1 diabetes mellitus. The determination of the glucose level was carried out in the capillary blood by means of the automatic glucose analyzer. For all the patients the glycemia was controlled in an hour after the injection of 0.1 unit/kg/h of insulin and then the dose was corrected in case of necessity with the control of the glucose level. For the treatment the fast-acting insulin was used and analogues of ultra fast-acting insulins, their total dose was calculated during the first day of treatment. Results. The glycemia level during the hospitalization fluctuated from 6.7 mmol/l to 62.2 mmol/l, at average it was 23.37 ± 10.16 mmol/l. 13 % of the patients had the glycemia of 13 mmol/l. 29 % of patients had the glycemia to 20 mmol/l, and 58 % of patients had the hyperglycemia of more than 20 mmol/l. 63,6 % of patients had the desirable dynamics of glycemia level in an hour after the injection of the calculated insulin dose, the glycemia decrease within an hour was 0.5–5.3 mmol/l, but it was noted the individual sensitivity to the injected insulin dose. The daily insulin dose fluctuated from 0.5 unit/kg daily to 5.83 unit/kg a day. The median of the daily insulin dose amidst the patients was 1.36 unit/kg and more often it was 0.92–1.88 unit/kg a day (25 %; 75 %). Conclusions. The insulin dose calculated as 0.1 unit/kg/h is efficient in most cases but due to the individual sensitivity to the injected insulin and the significant factor of the treatment efficiency is the glycemia control hourly for the opportune correction of the insulin dose.
Introduction
Diabetes mellitus (DM) refers to the most widespread chronical diseases of the mankind. The history of diabetology can be divided nominally into pre-insulinic and post-insulinic period. Actually the insulin discovery was a revolutionary breakthrough in the treatment of DM and in the changes of the possibilities and everyday life of the patients with DM [1, 2]. The insulin preparations and insulin introduction means have been improving at a quick rate, the insulin therapy technique and modes have been advancing. Thus, the life quality of patients has been improving and mortality due to the acute diabetes decompensation — diabetic ketoacidosis — has been decreasing. At the present phase of the diabetology development the gene engineering human insulins, the insulin analogues, insulin biosimilars are used. The insulin is administered by insulin syringe, pen injection device, insulin pump or syringe pump. Since the insulin deficiency is a releaser of all following metabolic changes in the patient’s organism with diabetic ketoacidosis (DKA), so the exact program of the insulin therapy is a guarantee of the effective treatment of the patient’s critical condition. The approaches to the DKA treatment have been changing together with the experience gained. It was time when in the insulin therapy the large insulin doses subcutaneously were used and it was used the sodium bicarbonate in the pathogenetic therapy. Now the approaches to the insulin therapy of the DKA were revised [3–6] and they can vary in case of the rare syndromes with the expressed insulin resistance manifestations [3].
In Ukraine the management protocols of patients with the DKA are represented in several documents, separately for patients with type 2 DM and type 1 DM. The first document approved by the Order of Health Ministry on December 21, 2012 № 1118 «Unified clinical protocol of the primary and secondary (specialized) medical assistance for type 2 diabetes mellitus» indicates the pre-hospital care in a volume of fast-acting insulin of 20 unit intramuscular; 0.9 % solution of sodium chloride intravenous (IV) by drop infusion at a speed of 1 l/h. At hospitals the mentioned document recommends to provide insulin therapy with small doses of fast-acting insulin (SEI) intravenous bolus injection, and then IV by drop infusion, taking into consideration the blood glucose level: with glycemia of 17–39 and higher — 0.1 unit/kg/h; with glycemia of 11 to 17 — 0,05 unit/kg/h; with glycemia less than 11 — 4–6 unit subcutaneously every 3–4 hours together with 5 % glucose solution. The insulin solution preparation: 50 unit of fast-acting insulin + 2 ml 20 % albumin solution (to avoid the adsorption of insulin molecules) for every 100 ml dilute to 500 ml 0.9 % sodium chloride, and we obtain the concentration of insulin solution 1 unit in 10 ml. The speed of glycemia decrease is not more than 4 mmol/l/h. In case of the absence of the decrease du–ring first 2–3 hours, the next dose of fast-acting insulin must be doubled (to 0.2 unit/kg/h), the hydration validity must be checked. In case of the decrease at about 4 mmol/l for an hour, the next dose of fast-acting insulin must be decreased twice (0.05 unit/kg/h).
The other document approved by the Order of Health Ministry № 254 of 27.04.2006 in Kyiv «About ratification of protocols of the medical assistance for children» indicates in the chapter about insulin therapy that «It must be done only after a successful getting out of shock and the beginning of the rehydration and administration of solutions containing potassium (because the penetration of potassium from blood plasma to cells can cause the cardiac arrhythmia). Insulin (only of short effect) is administrated with small doses continuously by drops or dissolved in 0.9% NaCl (1 unit/ml) by means of insulin pump. Before the insulin administration of 50 units it is dissolved in 50 ml of 0.9% NaCl, and 1 ml of this solution contains 1 unit of insulin. It is recommended the initial dose of 0.1 unit/kg/h (the youngest patients can receive 0.05 unit/kg/h). In case of the absence of positive dynamics of glycemia indices during first 2–3 hours the insulin dose should be doubled. The rate of glycemia decrease must be slow — not faster than 4–5 mmol/l per 1 hour. During the first day of the treatment the glycemia should not be reduced less than 13 mmol/l because the fast glycemia reduction can cause the cerebral edema. During the treatment the glycemia must be retained within 8–12 mmol/l. After the glycemia decreases to 14 mmol/l and in case of the normal indices of acid-base balance it is possible to proceed to insulin administration subcutaneously. The insulin therapy must not be stopped or reduced to the dose less than 0.05 unit/kg/h because it is necessary to ketogenesis inhibition and elimination of acidosis. That’s why the first subcutaneous injection is done 30 min. before the interruption of the IV administration. During the treatment the glycemia may decrease due to the improvement of renal perfusion and loss of the glucose with the urine, as well as the improvement of tissue perfusion with the improvement of inulin sensitivity». In the protocol developed but not approved by Health Ministry of Ukraine for the children with DKA of 2013 it was emphasized again the regime of small doses IV, explaining that insulin must be administered in 1-2 hours after the rehydration beginning, i.e. after the patient’s administration of the initial volume of the liquid. The dose like in the previous document is 0.1 unit/kg/h. But then it is pointed out that it is not recommended to administrate the fast-acting insulin via bolus injection because of the risk of cerebral edema. Then it is not recommended to leave the dose on the level of 0.1 unit/kg/h till the patient’s exit from DKA (рН > 7.3, bicarbona–tes > 15 mmol/l and/or the normalization of the anion gap). If a patient has the hypersensitivity to insulin it is possible to decrease a dose to 0.05 unit/kg/h or less providing that the metabolic acidosis continues decreasing. That time the appropriate rate of glycemia decrease after the beginning of insulin therapy was considered to be 2–5 mmol/l/h depending on the injection schedule and the amount of the glucose injected. In order to prevent the hypoglycemia it was recommended to add 5% glucose or 10% glucose with the further insulin infusion for the correction of metabolic acidosis. Also it was noticed that in case of the fast glycemia reduction (> 5 mmol/l/h) it was relevant to add the glucose to infusion solutions even before its concentration decreases to 17 mmol/l. This document studied the possible alternative way of insulin administration, in particular subcutaneous or intramuscular administration of analogues of the fast-acting insulin (lispro or aspart) every hour or every 2 hours at a dose subcutaneously: 0.3 unit/kg, in an hour and then every hour — insulin lispro or aspart calculated as 0.1 unit/kg or 0,15–0,20 unit/kg every 2 hours. If the concentration of the blood glucose falls < 14 mmol/l in order to prevent DKA (рН < 7.3) it is necessary to add 5% glucose and continue the insulin therapy as mentioned above. When the diabetic ketoacidosis signs decrease, glycemia is < 14 mmol/l, the dose of insulin lispro or aspart insulin should be reduced to 0.05 unit/kg/h in order to retain the blood glucose on the level of ≈11 mmol/l till the complete elimination of the DKA.
The next document approved by the Order of the Health Ministry of Ukraine of December 29, 2014, № 1021 (Unified clinical protocol of the primary, emergency, secondary (specialized) and tertiary medical assistance) also recommends small doses. But it also indicates the bolus insulin IV injection at the beginning of the insulin therapy. The doses of fast-acting insulin are recommended to inject taking into conside–ration the level of the blood glucose: having glycemia 17–39 mmol/l and more — 0.1 unit/kg/h; having glycemia from 11 to 17 mmol/l — 0.05 unit/kg/h; having glycemia less than 11 mmol/l — it is recommended to proceed with 4-units subcutaneously every 3–4 hours adding the infusion of 5% glucose solution. The preparation of the insulin solution is described above with the addition of the albumin. The recommended rate of the glycemia reduction is not more than 4 mmol/l/h. Hereafter it was recommended to change the insulin dose depending on the glycemia dynamics in an hour: to increase the dose twice in case of the absence of the glycemia decrease during 2–3 hours, and in case of the glycemia decrease it must be controlled every hour.
Analyzing the documents and literature mentioned above [6–11], some questions arise:
— Is it necessary to use the strictly determined intramuscular injection of insulin at a dose of 20 units for all the patients with type 2 DM at the pre-hospital stage?
— Is it necessary the bolus insulin dose IV at the beginning of the intensive therapy at hospitals [8–16]?
— What is the initial dose of the insulin for the continuous intravenous insulin infusion by means of syringe pump [8–16]?
— Are there any priorities for the usage of gene engineering fast-acting insulin or analogue of super
fast-acting insulin in the intensive therapy of the DKA?
— Will the usage of insulin infusion without albumin addition be effective [16]?
— How fast must we begin the insulin solution infusion — at once or in 1–2 hours after the rehydration?
— What is the expected rate of the glycemia reduction?
— What daily dose of insulin calculated for the body mass will be effective on the first day of the intensive treatment of the critical condition?
It is known that the main criteria of the laboratory diagnostics and the determination of the severity of the DKA in most countries are the glucose level, blood рН and blood bicarbonates level. However, analyzing the data of the literature for the recent years, the glycemia level as the diagnostic criterion was revised and reduced from 13.9 mmol/l to 11 mmol/l, and the recent scientific publications claim about the high frequency of the euglycemic ketoacidosis using new groups of sugar-lowering preparations or restricting carbohydrates intake with meals [7]. But the рН level and blood bicarbonates are constant within decades as indices of the severity of ketoacidosis [2–6]. Precisely the level of the glycemia in dynamics is the evaluation of the insulin therapy efficacy, however, only the figures of the glycemia are not important without other clinical semiotics.
The purpose of the study — the evaluation of the insulin therapy efficacy for the DKA with syringe pump by means of the continuous infusion with the initial dose 0.1 unit/kg/h under the control of the glycemia as the principal component of the pathogenetic treatment and the determination of the necessary daily insulin dose on the first day of the DKA treatment in parallel with the rehydration start by means of physiological solution.
Materials and methods
The research group consisted of 55 patients with DM who were hospitalized urgently in the condition of the DKA into the department of the intensive therapy and anesthesiology of Vinnytsia Regional Endocrinological Centre during 2009–2014. The patients were included into the research group intermittently at random. There were information cards for every patient. The cards reflect the detailed list of complaints (in case of unconscious patient the complaints were written according to relatives), the data of objective examination, additional examination and treatment methods.
The criteria of admission of patients were the pre–sence of clinical semiotics of the DKA and рН level of venous blood (< 7.35 mmol/l) and blood bicarbonates (< 15 mmol/l), ketonuria. The criteria of rejection were the children with the weight less than 20 kg, the advanced stage of the renal and hepatic failure. The acute complications of DM at the stage of the ketoacidosis were diagnosed according to the clinical symptoms accor–ding to the protocol of the medical assistance, approved by the Order of Health Ministry of Ukraine № 254 of 27.04.2006 and Unified clinical protocol of the primary and secondary (specialized) medical assistance for the type 2 diabetes mellitus approved by the Order of Health Ministry of Ukraine № 1118 of 21.12.2012.
The age of the patients with DKA was from 9 to 65 years. The average age of the patients was 31.58 ±
± 17.18 years. The specific weight of the female patients was 56 %, and male patients — 44 %. 44 patients (80 %) had type 1 DM, and 11 patients (20 %) had type 2 DM. Analyzing the age structure of patients with DKA it was revealed the biggest number of patients (67.27 %) at the age of 19–40 years old and 41–60 years old that corresponds to the group of labour pool. The next frequent category was children (21.83 %) among whom there were more children of adolescence (18.18 %). But the aged people were the least.
All examined patients were divided into 3 groups according to the indices of the acid base balance (Wolfsdorf J.I., 2009): the patients with the minor ketoacidosis of the І stage, the patients with the moderate DKA of the ІІ stage and the patients with severe DKA of the ІІІ stage. Simultaneously the patients were divided according to the severity by the clinical symptoms.
The patients’ examinations were executed using the clinical, biochemical and instrumental methods of the research. The general clinical examination was performed according to the common methods. Clinical and laboratory trials were executed in compliance of all ethic norms after the informed consent for examination and treatment.
The biochemical blood indicators were determined by the biochemical semiautomatic photometer-analy–zer Microlab 300 (manufactured by “Vital Sciontific”, Netherlands, 2005). Glycated hemoglobin (HbA1c) was determined from the venous blood by the chromatographic method on the analyzer D-10 manufactured by “Віо-Rad” (France, 2005). Electrolytic composition of the venous blood, in particular the concentration of potassium, sodium, calcium was determined by means of the automatic analyzer Easy lite (USA, 2005). The level of urine ketones was determined by the qualitative method by means of test-strips “Ketofen” LACHEMA. All the patients did the blood analysis to determine the gas composition and indices of acid-base balance by the device “Еаsy Blood Gas” (USA, 2008). The glucose level determination on the principle of electrochemical measurement was carried out by the biosensor in the fresh capillary blood by means of the automatic glucose and lactate analyser Super GL ambulance, № 0381, № 0380 (the manufacturer is the company “Dr. Müller Gerätebau GmbH”, Germany, 2006, 2008). The function study included the electrocardiograms monitoring, the plan radiography of thoracic organs, ultrasound examination of internal organs.
The findings were processed statistically using the Russian language program Statistica 6.1 StatSoft, 1995. Taking into consideration that during the analysis of the initial data in most cases the distribution was not normal, the distribution-free statistical methods were applied. The median, minimum value, maximum value, interquartile range from 25 % to 75 % was determined. The certainty of values difference between independent qualitative values in paired samples was determined by means of Mann-Whitney U-test. It was calculated Spearman’s correlation coefficient between the necessary insulin dose and the ketoacidosis depth. The critical level of significance was the level 0.05.
Results
From the clinical point of view among the patients with ketoacidosis three sequential stages of DKA were distinguished: І — moderate ketoacidosis, ІІ — precoma or uncompensated ketoacidosis, ІІІ — coma stage [3, 12]. Considering clinical symptoms (according to the clinical picture above described) during the hospitalization in the anesthesiology department it was revealed that 8 persons had the DKA of the І stage, 41 persons — the ІІ stage, 6 persons — the ІІІ stage. Taking into consideration that the discriminate criterion was the blood рН level, the patients’ distribution took place in other way. 23 persons had the DKA of І stage revealed according to the venous blood рН level, 12 — with ІІ stage, 20 persons with ІІІ stage. Accor–ding to the blood рН level the patients were hospitalized with the same frequency in the condition of the minor or severe ketoacidosis, but the moderate ketoacidosis was almost twice more rarely. On the contrary, considering the clinical picture of the ketoacidosis indisputable majority of patients with DKA (74.55 %) were the patients with moderate severity and the frequency of ketoacidosis coma (10.90 %) was the least. The main indices obtained of the venous blood of the patients with DKA are given in the table 1.
/65-1.jpg )
The values of the acid-base balance (blood рН and bicarbonates) differed statistically during different severity grades of DKA (р < 0,001). On the contrary the glycemia level differed statistically differed statistically only between the patients of minor and severe ketoacidosis.
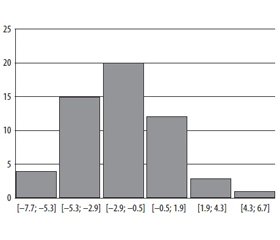
The classical picture of DKA is characterized with hyperglycemia more than 13 mmol/l, but there is so called euglycemical ketoacidosis providing the restriction of carbohydrates intake with meals or usage of glyflasides [7]. Only 13 % of our patients had the glycemia of 13 mmol/l. 29 % of patients had the glycemia of 13–20 mmol/l, and 58 % of patients had the hyperglycemia of more than 20 mmol/l. The glycemia level among the patients fluctuated from 6.7 mmol/l to 62.2 mmol/l. The histogram of the glycemia level distribution among the patients with DKA during the hospitalization with the pace in 9.4 mmol/l is given in the Figure 1.
The bolus insulin dose was not administrated at the beginning of the treatment [4, 12–14] because the insulin circulates in the blood after the administration only during 7 minutes although some literature data justify its application [8, 16, 17]. The initial dose was admini–strated calculated as 0.1 unit/kg/h. The recommended dose for young children calculated as 0.05 unit/kg was also applied in the practice but with young children of the weight less than 20 kg not included into the trial. The calculated dose was administrated in parallel with the physiological solution infusion via continuous intravenous administration by syringe pump. However the expected dynamics of glycemia level in an hour was not the same.
Never once we added albumin to insulin solution to prevent the absorption of the biological preparation by the system. In 30–60 min. after the consequent insulin injection via syringe pump we obtained the insulin action without using the expensive protein preparation. So we also consider unreasonable to use albumin.
The obtained data are given in the Figure 2.
In the trial for the treatment of the DKA there were used both gene engineering insulins of different manufacturers and the analogues of super fast-acting insulins. The priorities for certain types of the insulin were not determined. We always were aimed to the insulin type which the patient received before the critical decompensation. Taking into account its usual daily dose it was possible to forecast the sensitivity to the initial dose injected. During the first indication gene engineering fast-acting insulins available at the hospital were used. Insulins used in the trial were the following: Humulin R, Humodar R, Humalog, Farmasulin N, Actrapid NM, Novorapid, Insuman Rapid.
As the figure 2 shows the dynamics of the glycemia level after the calculated insulin dose is not the same. In an hour the sugar changed considerably: it both decreased by 7.7 mmol/l and increased by 6.7 mmol/l. But totally in most cases it was reduced at a desirable level — 0.5–5.3 mmol/l. The usage of intravenous insulin without initial bolus dose calculated as 0.1 unit/kg/h allowed in 63.6 % to achieve the desirable reduction of glycemia already in an hour. However not all the patients had the expected biological effect with the mentioned dose. 7.3 % of cases noted more critical glycemia reduction but with the appropriate correction of the dose this ”insulin super effect”, or rather, the individual sensitivity to insulin did not worsen the treatment efficacy. 7.3 % of patients had the glycemia level risen at 1,9–6,7 mmol/l after the initial dose injection. 21.8 % of patients had the glycemia level within ± 1 mmol/l in an hours after the beginning of the insulin therapy. Of course, the sharper decrease was noted in case of the higher original glycemia level. In case of the sharp reduction of the blood glucose the dose was reduced twice and then it was corrected constantly under the glycemia control every 30–60 min. till the achievement of the stable glycemia dynamics and positive clinical dyna–mics. If the blood glucose increased, the initial dose was increased 50–100 %. Such tactics gave the possibility to achieve the desirable glycemia level.
The daily insulin dose (the sum of the injected
insulin by the syringe pump daily) fluctuated from 0.5 unit/kg daily to 5.83 unit/kg a day. The median of the daily insulin dose amidst the patients was 1.36 unit/kg a day, more often it was 0.92–1.88 unit/kg a day (25 %; 75 %). Evaluating the daily insulin dose with different clinical courses of the DKA, different doses were obtained but they did not differ very much statistically. The highest doses were during the encephalopathic (the median 1.59 unit/kg a day) and nephrotic (2.55 unit/kg a day) courses combined with the changes of the blood potassium level and correspon–dingly in the tissues. The patients with gastroenterological course of DKA (the median 1.30 unit/kg a day) had the lowest daily insulin dose.
Discussion
Since the patients were delivered to the tertiary level of the medical care by the ambulance crew the primary medical assistance was assessed always. All the patients with type 2 DM were not injected 20 units of fast-acting insulin intramuscularly. During the following course of DKA with the right tactics of the management of the patient with type 2 DM the absence of this dose did not influence, because in our opinion without the differentiation for the body mass and insulin sensitivity, without the consideration of the different insulin absorption rate this injection way and injection dose is not reasonable. No recommendation to inject intramuscularly 20 units at the pre-hospital stage was found in the foreign lite–rature [3–6, 11–16], except the recommendations of the medical specialized assistance in the Russian Fede–ration [16] which still remain unchanged in 2017 [17]. For the determination of the initial dose it was calculated according to the method described above. In the literature randomized studies of the efficient dose for the treatment of DKA are rather scanty because the object of the study is the critical condition developing acutely. Nevertheless in general the initial dose with or without bolus dose is estimated, the calculation is done in general in the dose of 0.1 unit/kg/h, 0.14 unit/kg/h or 0.3 unit/kg/h in case of subcutaneous administration [5, 8, 11]. We chose the only way of insulin administration during the treatment of DKA, it’s intravenous way, because we thank that under the conditions of the defected microcirculation its absorption of subcutaneous tissue is worsened although some experts unite intravenous and subcutaneous ways of administration in spite of stating the renewal of ketoacidosis after the crossover to the subcutaneous insulin administration [3, 11, 12]. The initial insulin dose of 0.1 unit/kg/h gave the desi–rable effect of glycemia reduction in 63.8 % of cases, in 21.8 % — slow glycemia reduction, in other cases it was necessary to make correction: to decrease the dose in 7.3 %, to increase it in 7.3 %.
The average daily insulin dose for the treatment of DKA according to the literature data is 0.6–2 unit/kg (calculated for the body weight 100–300 units) [2, 8–10, 12], however there are rare cases of the insulin receptors pathology when the daily dose can reach 50000 units [3]. Most experts tend to small doses methods and to the calculation of the initial dose of 0.1 unit/kg/h, but they also claim about the possible considerable increase the calculated dose due to the individual insulin resistance. It is interesting to note that all the patients mentioned in the rare cases before the DKA received insulin contained in 1 ml 500 units which is not used yet in Ukraine, also before the acute decompensation of DM their daily dose was high. The majority of our patients had type 1 DM, the daily insulin dose was 0.9–2 unit/kg. All the patients received gene engineering insulin or analogue of insulin (1 ml = 100 units). Determining the correlation relationship of the daily insulin dose with blood рН it was established significant statistically (р < 0.05) negative dependence between the blood рН level and the insulin dose (Spearman’s cor–relation coefficient rs = –0.38) so with the increase of the DKA the daily insulin dose increased.
In general the insulin therapy together with the right chosen infusive therapy allows to decrease the clinical evidence of ketoacidosis during 8-12 hours of the treatment what was confirmed by laboratory data.
Conclusions
1. The administration of insulin intravenously via s syringe pump without the initial bolus dose calcula–ted as 0.1 unit/kg/h is efficient in most cases, however there is an individual sensitivity to the injected insulin and it provides both the glycemia increment and its fast reduction. Only providing hourly glycemia control for the opportune correction of the insulin dose this tactics permits to correct steadily and constantly the insulin deficit for the patients with the diabetic ketoacidosis.
2. The dominating majority of our examined patients had type 1 diabetes mellitus, the daily insulin dose was 0.9–2 unit/kg on the first day of treatment although its limits were wider.
3. Without albumin administration the injected insulin IV via syringe pump infusion acted already in an hour in spite of its possible absorption by polyvinylchloride fittings.
4. The usage of both fast-acting insulin and analogues of ultra fast-acting allows to compensate the key factor of the diabetic ketoacidosis — the progressing insulin deficiency — when properly chosen the insulin therapy method together with other components of the pathogenetic treatment of the critical condition of a patient.
Conflicts of interests. Authors declare the absence of any conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
Список литературы
1. Нікберг І.І. Що знали про цукровий діабет у стародавні часи? / І.І. Нікберг // Міжнародний ендокринологічний журнал. — 2013. — № 1(49). — С. 70-72.
2. Бицунов Н.С., Плохой А.Д., Шилова Н.А. Опасности, ошибки и осложнения на этапах проведения интенсивной терапии диабетической кетоацидотической комы / Н.С. Бицунов, А.Д. Плохой, Н.А. Шилова // Вестник интенсивной терапии. — 1998. — № 2. — С. 47-49.
3. Robinson C., Cochran E., Gorden P., Brown R. Management of Diabetic Ketoacidosis in Severe Insulin Resistance // Diabetes Care. — 2016. — Vol. 39. — P. 116-118. https://doi.org/10.2337/dc16-0635
4. Wolfsdorf J., Glaser N., Sperling M.A. Diabetic Ketoacidosis in Infants, Children, and Adolescents // Diabetes Care. — 2006. — Vol. 29. — P. 1150-1159. doi: 10.1111/pedi.12165.
5. Umpierrez G.E., Sidney J., Smiley D. et al. Insulin Analogs Versus Human Insulin in the Treatment of Patients With Diabetic Ketoacidosis // Diabetes Care. — 2009. — Vol. 32. — P. 1164-1169. doi: 10.2337/dc09-0169. PMCID: PMC2699711.
6. Umpierrez G.E., Cuervo R., Karabell A. et al. Treatment of diabetic ketoacidosis with subcutaneous insulin Aspart // Diabetes Care. — 2004. — Vol. 27. — P. 1873-1878. https://doi.org/10.2337/diacare.27.8.1873
7. Misaghian-Xanthos N., Shariff A.I., Mekala K. et al. Sodium-Glucose Cotransporter 2 Inhibitors and Diabetic Ketoacidosis: A Case Series From Three Academic Institutions // Diabetes Care. — 2017. — Vol. 40(6). — P. 65-66. https://doi.org/10.2337/dc16-2591
8. Шлапак І.П. Цукровий діабет: погляд з позиції лікаря-анестезіолога / І.П. Шлапак, О.А. Галушко. — К.: Книга плюс, 2010. — 160 с.
9. Науменко В.Г. Невідкладні стани в клініці цукрового діабету. Патогенез, клініка, діагностика та лікування діабетичної коми / В.Г. Науменко // Міжнародний ендокринологічний журнал. — 2006. — № 3(9). — С. 70-72.
10. Кравчун Н.А. Особенности лечения диабетического кетоацидоза в современных условиях / Н.А. Кравчун, И.В. Чернявская, А.В. Козаков // Біль, знеболювання і інтенсивна терапія. — 2010. — № 2. — С. 43-47.
11. Dyanne P. Diabetic Ketoacidosis: Evaluation and Treatment // American Family Physician. — 2013. — Vol. 87(5). — P. 337-346. PMID: 23547550.
12. Mazer M., Chen E. Is subcutaneous administration of rapid-acting insulin as effective as intravenous insulin for trea–ting diabetic ketoacidosis? // Ann. Emerg. Med. — 2009. — Vol. 53(2). — P. 259-263. PMID: 19177639.
13. Kitabchi A.E., Murphy M.B., Spencer J. et al. Is a pri–ming dose of insulin necessary in a low-dose insulin protocol for the treatment of diabetic ketoacidosis? // Diabetes Care. — 2008. — Vol. 31(11). — P. 2081-2085. PMCID: PMC2571050. doi: 10.2337/dc08-0509.
14. Wilson J.F. Diabetic ketoacidosis // Ann. Intern. Med. — 2010. — Vol. 152(1). — P. 1-15. doi: 10.7326/0003-4819-152-1-201001050-01001. PMID: 20048266.
15. Wolfsdorf J., Craig M.E., Daneman D. et al. Diabetic ketoacidosis in children and adolescents with diabetes // Pediatr. Diabetes. — 2009. — Vol. 10(12). — P. 118-133. doi: 10.1111/j.1399-5448.2009.00569.x.
16. Дедов И.И., Шестакова М.В., Александров А.А. и др. Алгоритмы специализированной медицинской помощи больным сахарным диабетом / Под ред. И.И. Дедова, М.В. Шестаковой. — 6-й выпуск. — 2013. — T. 16. — C. 28-31.
17. Дедов И.И., Шестакова М.В., Майоров А.Ю. и др. Алгоритмы специализированной медицинской помощи больным сахарным диабетом / Под ред. И.И. Дедова, М.В. Шестаковой. — 8-й выпуск. — 2017. — T. 20. — C. 29-33.

/65-1.jpg )
/66-1.jpg )