Резюме
Актуальність. Зазвичай вважають, що фактори навколишнього середовища та генетичні фактори взаємодіють з утворенням фенотипу неалкогольної жирової хвороби печінки (НАЖХП) та визначають її прогресування. Як НАЖХП, так і цукровий діабет 2-го типу (ЦД2) становлять собою гетерогенні захворювання зі спільними патогенетичними шляхами. Адипонектин — це адипокін, що підвищує чутливість гепатоцитів та м’язів до інсуліну, модулює енергетичний гомеостаз, глюкозний/ліпідний метаболізм та запальну відповідь. Ряд значущих поліморфізмів гена адипонектину відомі в цієї галузі. Метою дослідження була оцінка можливої асоціації між двома варіантами гена адипонектину (ADIPOQ) — +276 G/T (rs1501299) і –11391 G/A (rs17300539) та чутливістю до НАЖХП у хворих на ЦД2 української популяції. Матеріали та методи. Дослідження за типом «випадок — контроль» включало 155 хворих на ЦД2 (ч./ж. — 77/78, вік — 54,55 ± 0,73 року, тривалість діабету — 6,66 ± 0,49 року, індекс маси тіла — 32,20 ± 0,43 кг/м2, обсяг талії/стегон — 0,98 ± 0,01 м, HbA1c — 7,26 ± 0,11 %) для біохімічної характеристики (ліпідний профіль, неестерифіковані жирні кислоти (НЕЖК), інсулін, загальний адипонектин та ін.), у тому числі 90 хворих на ЦД2 із НАЖХП, 245 хворих на ЦД2 для генотипування rs1501299, 155 хворих на ЦД2 для генотипування rs17300539 та 51 контрольну особу відповідного віку (К). Варіанти +276 G/T та –11391 G/A визначали за допомогою полімеразної цепної реакції — довжини рестрикційних фрагментів з ендонуклеазами Mva1269I (BsmI) та MspI (HpaII). Інсулінорезистентність (ІР) оцінювали за допомогою алгоритму HOMA та як жирову ІР (адипо-ІР, НЕЖК × інсулін). Для статистичного аналізу використовували непарний t-критерій Стьюдента, χ2 та ранговий критерій Спірмана. Для оцінки генетичного ризику НАЖХП розраховували відношення шансів та 95% довірчий інтервал (ДІ). Результати. Хворі на ЦД2 характеризувалися надлишковою масою тіла або ожирінням, що було більш вираженим за наявності НАЖХП
(p < 0,01). Це супроводжувалося підвищенням рівня НОМА-ІР (p < 0,05) та тригліцеридів (p < 0,001). Відмічено, що адипо-ІР була вищою у хворих на ЦД2 порівняно з К (p < 0,001) та цей індекс суттєво підвищений у хворих на ЦД2 із НАЖХП порівняно з хворими на ЦД2 без НАЖХП такого ж ступеня ожиріння (190,18 ± 22,15 проти
133,32 ± 13,58 ммоль/л · пмоль/л, p < 0,02) з оберненою кореляцією між адипо-ІР та рівнем адипонектину тільки у хворих на ЦД2 із НАЖХП (rs = –0,350, p = 0,021). Стратифікація хворих без НАЖХП за генотипом +276 G/T свідчить про поширеність GT- та TT-генотипів. Таким чином, G-алель rs1501299 підвищує ризик розвитку НАЖХП порівняно з T-алелем (OR = 4,44, 95% ДІ = 2,89–6,81,
p < 0,05). Ми також виявили значущу різницю в частотах –11391G/A між хворими на ЦД2 та К, але не між хворими з НАЖХП та без неї. Ми спостерігали, що гаплотип GT/GG частіше зустрічався серед хворих на ЦД2 із НАЖХП та удвічі менше в пацієнтів без хвороби печінки (33 та 16,49 % відповідно, p < 0,05). Висновки. Обґрунтовано перспективність використання індексу адипо-ІР як предиктивний маркер розвитку НАЖХП та індикатор успішності терапії хворих на ЦД2. Виявлено новітні генетичні маркери (G-алель rs1501299, GG/GG- та GT/GG-гаплотипи за rs17300539 та rs1501299 відповідно) для ризику розвитку НАЖХП у хворих на ЦД2.
Актуальность. Обычно полагают, что факторы окружающей среды и генетические факторы взаимодействуют с формированием фенотипа неалкогольной жировой болезни печени (НАЖБП) и определяют ее прогрессирование. Как НАЖБП, так и сахарный диабет 2-го типа (СД2) представляют собой гетерогенные заболевания с общими патогенетическими путями. Адипонектин — это адипокин, повышающий чувствительность гепатоцитов и мышц к инсулину, модулирующий энергетический гомеостаз,
глюкозный/липидный метаболизм и воспалительный ответ. В данной области известен ряд значимых полиморфизмов гена адипонектина. Целью исследования была оценка возможной ассоциации между двумя вариантами гена адипонектина (ADIPOQ) — +276 G/T (rs1501299) и –11391 G/A (rs17300539) и чувствительностью к НАЖБП у больных СД2 украинской популяции. Материалы и методы. Исследование по типу «случай — контроль» включало 155 больных СД2 (м./ж. — 77/78, возраст — 54,55 ± 0,73 года, длительность диабета — 6,66 ± 0,49 года, индекс массы тела — 32,20 ± 0,43 кг/м2, объем талии/бедер — 0,98 ± 0,01 м, HbA1c 7,26 ± 0,11 %) для биохимической характеристики (липидный профиль, неэстерифицированные жирные кислоты (НЭЖК), инсулин, общий адипонектин и др.), в том числе 90 больных СД2 с НАЖБП, 245 больных СД2 для генотипирования rs1501299, 155 больных СД2 для генотипирования rs17300539 и 51 контрольное лицо соответствующего возраста (К). Варианты +276 G/T и –11391 G/A определяли с помощью полимеразной цепной реакции — длины рестрикционных фрагментов с эндонуклеазами Mva1269I (BsmI) и MspI (HpaII). Инсулинорезистентность (ИР) оценивали с использованием алгоритма HOMA и как жировую ИР (адипо-ИР, НЭЖК × инсулин). Для статистического анализа применяли непарный t-критерий Стьюдента, χ2 и ранговый критерий Спирмана.
Для оценки генетического риска НАЖБП рассчитывали отношение шансов и 95% доверительный интервал. Результаты. Больные СД2 характеризовались избыточной массой тела или ожирением, более выраженным при НАЖБП (p < 0,01). Это сопровождалось повышением уровня НОМА-ИР (p < 0,05) и триглицеридов (p < 0,001). Выявлено, что адипо-ИР была выше у больных СД2 по сравнению с К (p < 0,001) и этот индекс существенно больше у больных СД2 с НАЖБП по сравнению с больными СД2 без НАЖБП с сопоставимой степенью ожирения (190,18 ± 22,15 против 133,32 ± 13,58 ммоль/л · пмоль/л,
p < 0,02) с отрицательной корреляцией между адипо-ИР
и уровнем адипонектина только у больных СД2 с НАЖБП (rs = –0,350, p = 0,021). Стратификация больных без НАЖБП по генотипу +276 G/T свидетельствует о повышенной частоте встречаемости GT- и TT-генотипов. Таким образом, G-аллель rs1501299 повышает риск развития НАЖБП по сравнению с T-аллелем (OR = 4,44, 95% доверительный интервал = 2,89–6,81, p < 0,05). Мы также выявили значимое различие в частотах –11391G/A между больными СД2 и К, но не между больными с НАЖБП и без нее. Мы обнаружили, что гаплотип GT/GG встречался чаще у больных СД2 с НАЖБП и вдвое реже у пациентов без болезни печени (33 и 16,49 % соответственно, p < 0,05). Выводы. Обоснована перспективность использования индекса адипо-ИР в качестве предикторного маркера развития НАЖБП и индикатора успешности терапии больных СД2. Выявлены новые генетические маркеры (G-аллель rs1501299, GG/GG- и GT/GG-гаплотипы по rs17300539 и rs1501299 соответственно) для риска развития НАЖБП у больных СД2.
Background. It is generally believed that environmental and genetic factors interact with the formation of nonalcoholic fatty liver disease (NAFLD) phenotype and determine its progression. Both NAFLD and type 2 diabetes (T2D) are heterogeneous diseases with common pathogenic pathways. Adiponectin is an adipokine, which increases the sensitivity of hepatocytes and muscle to insulin, modulates energy homeostasis, glucose/lipid metabolism, and inflammatory response. A number of significant adiponectin gene polymorphisms are known in this area. The purpose of the study was to evaluate the possible association between two adiponectin gene (ADIPOQ) variants, +276 G/T (rs1501299) and –11391 G/A (rs17300539), and susceptibility to NAFLD in T2D patients of Ukrainian population. Materials and methods. Case-control study included a total of 155 persons with T2D (males/females: 77/78, age 54.55 ± 0.73 years, T2D duration 6.66 ± 0.49 years, body mass index 32.20 ± 0.43 kg/m2, waist/hip circumference 0.98 ± 0.01 m, HbA1c 7.26 ± 0.11 %) for biochemical characteristics (lipid profile, non-esterified fatty acids (NEFA), insulin, total adiponectin, etc.), including 90 T2D patients with NAFLD, 245 — with rs1501299 genotyping, 155 — with rs17300539 genotyping, and 51 sex and age-matched control subjects. The +276 G/T and –11391 G/A were determined by polymerase chain reaction — restriction fragment length polymorphism method with endonucleases Mva1269I (BsmI) and MspI (HpaII). Insulin resistance (IR) was assessed using homeostasis model assessment (HOMA) algorithm and as adipose IR (Adipo-IR, NEFAxinsulin). Unpaired Student’s t test, χ2 test and Spearman’s rank order were used. To predict the probabilities of genetic risk in NAFLD, the odds ratio (OR) and 95% confidence interval (CI) were calculated. Results. T2D patients were characterized by overweight and obesity, which were more significant in the presence of NAFLD (p < 0.01). It was accompanied by an increase in НОМА-IR (p < 0.05) and triglycerides (p < 0.001) levels. We found that Adipo-IR was higher in patients with T2D as compared to the controls (p < 0.001), and this index was significantly increased in T2D patients with NAFLD in contrast to obesity-matched persons without NAFLD (190.18 ± 22.15 vs 133.32 ± 13.58 mmol/L·pmol/L, p < 0.02), with negative correlation between Adipo-IR and adiponectin level in T2D patients with NAFLD only (rs = –0.350, p = 0.021). Stratification of non-NAFLD patients by +276G/T genotype suggests the prevalence of GT- and TT-genotypes. Thus, the rs1501299 G-allele increased the risk of NAFLD in comparison with T-allele (OR = 4.44, 95% CI = 2.89–6.81, p < 0.05). We also found a significant difference in the frequency of –11391G/A between T2D and control groups, but not between the patients with and without NAFLD. We observed that the haplotype of GT/GG had been more common in T2D with NAFLD, and twice less often detected in patients without hepatic disease (33 and 16.49 %, respectively, p < 0.05). Conclusions. We can recommend Adipo-IR index as a predictive marker for the NAFLD development and the indicator for therapy success in T2D patients. We established new genetic markers (rs1501299 G-allele, rs17300539 and rs1501299 GG/GG and GT/GG haplotypes, respectively) for the risk of NAFLD development in T2D patients.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is closely related with visceral obesity and insulin resistance (IR) [1], which are early and powerful determinants of type 2 diabetes mellitus (T2D). Although NAFLD is not one of the defining criteria for metabolic (IR) syndrome, it is a common hepatic manifestation [2]. On the other hand, L. Amedeo et al. (2015) are thought, that the conventional paradigm of NAFLD representing the “hepatic manifestation of the metabolic syndrome” is outdated [1]. However, NAFLD is usually associated with obesity or IR and thus can be regarded as sign of the metabolic syndrome. It should be looked for in patients with obesity, diabetes or lipid disturbances [3]. Dyslipidemia, IR, increased production of proinflammatory cytokines, low adiponectin, high PAI-1 levels, hypertension, and hyperglycemia are main factors that lead to NAFLD, further aggravate the course of NAFLD, and accelerate the progress of atherosclerosis and the development of cardiovascular disease (CVD) [4]. The high prevalence of T2D, about 2 % of the world’s population [5], the specificity of clinical manifestations and the life quality of these patients make the research of this disease and its complications especially urgent. On the other hand, obesity, hyperglycemia, T2D and hypertriglyceridemia are known as risk factors for the NAFLD development [6–8]. It is generally believed that environmental and genetic factors interact to produce NAFLD phenotype and determine its progression [9]. Both NAFLD and T2D are heterogeneous diseases. Common pathways involved in the pathogenesis of NAFLD and T2D include IR, atherogenic dyslipide–mia, subclinical inflammation, oxidative stress, CVD, chronic kidney disease, and obesity [10].
Recent studies have pointed role of adipose tissue as an active endocrine organ that can secrete a number of specific adipokines (including chemokines, cytokines and hormones) which can affect energy balance and endocrine mechanisms responsible for regulating appetite, lipid metabolism, blood pressure, insulin sensitivity, and systemic inflammation. An important role in this system plays adiponectin [5]. Adiponectin is an adipose tissue-specific plasma protein produced in growing adipocytes, prevents the formation of IR syndrome, increases the sensitivity of hepatocytes and muscle to insulin, possesses a protective effect on the vascular wall, modulates insulin sensitivity, energy homeostasis, glucose and lipid metabo–lism, and anti-inflammatory responses in the vascular system [11, 12]. However, as soon as fat tissue increases in volume, adiponectin concentration is reduced [13], and the low level of this hormone is combined with the further development of obesity-related disturbances [14].
Adiponectin shows protective properties in alcoholic and nonalcoholic fatty liver disease [15]. In liver, adiponectin attenuates IR by increasing of insulin sensitivi–ty [16]. At present time, however, the molecular mechanisms underlying the development and the progression of NAFLD are poorly understood. To clarify the nature of the link between the gene and metabolic state of the organism, ADIPOQ SNPs are investigated, among others
[17]. Adiponectin is encoded by ARM1 (ADIPOQ, ACDC) gene, located on the long arm of chromosome 3 locus at 3q27 [18, 19], which covers 16 kb and contains three exons and two introns. The results of genome wide study of the locus 3q27 identified its relationship with susceptibility to diabetes [20, 21]: it is associated with T2D and metabolic syndrome in Japanese [21], USA [22], French (Caucasoids) [19] and Italian [23] populations. We have previously identified association of adiponectin gene (ADIPOQ) SNP +276 G/T and paraoxonase-1 gene (PON-1) SNP Q192R with the risk of T2D development in Ukrainian population [24, 25].
The purpose of the present study was to evaluate the possible association between two adiponectin gene variants, +276 G/T (rs1501299) and –11391 G/A (rs17300539), and susceptibility to NAFLD in type 2 diabetic subjects of Ukrainian population.
Materials and methods
Case-control study included a total of 155 patients with a diagnosis of T2D (M/F: 77/78, age 54.55 ± 0.73, T2D duration 6.66 ± 0.49 yrs, body mass index (BMI) 32.20 ± 0.43 kg/m2, waist-to-hip ratio (WHR) 0.98 ± 0.01, HbA1c 7.26 ± 0.11 %) including 90 T2Ds with NAFLD and 51 sex and age-match control subjects (C) for biochemical characteristics, 245 T2Ds and 103 C for rs1501299 genotyping, 155 T2Ds and 51 C for rs17300539 genotyping. The data were collected through a standard questionnaire. All patients were interviewed regarding a full medical history that included age, sex, occupation, duration of diabetes, mode and duration of treatment, presence of any associated illness, surgical history, personal history of smoking/alcohol/drug abuse, dietary habit and family history of diabetes. Controls were individuals with no clinically significant abnormal physical findings. None of the controls had any personal history of diabetes at the time of blood donation, which was ascertained with a questionnaire completed by each healthy volunteer. All cases and controls signed an informed consent for clinical, biochemical and genetic studies and the protocols were approved by the institutional review board of SI “V. Danilevsky Institute of Endocrine Pathology Problems of NAMS of Ukraine”.
Cases and controls from study were all Caucasoids and residents of Kharkiv region (Ukraine). The cases were clinically and biochemically confirmed as T2D. The diagnosis NAFLD was verified in accordance with the recommendations of the American Gastroenterolo–gical Association (AGA) and the American Association for the Study of liver disease based on the clinical course of the disease, lipid and carbohydrate metabolism, activity of alaninaminotransferase (ALT), aspartataminotransferase (AST), ratio ALT/AST and sonographic examination [26]. Nutritional status was assessed by measuring weight, height and abdominal circumference using well established techniques.
In all the ADIPOQ SNP +276 G/T (rs1501299) and ADIPOQ SNP –11391 G/A (rs17300539) were determined by polymerase chain reaction (PCR) — restriction fragment length polymorphism method (RFLP) with endonucleases Mva1269I (BsmI) and MspI (HpaII). Pri–mers for ADIPOQ SNP +276G/Т were as follows: Forward
(ADIPOQ276F GGCCTCTTTCATCACAGACC) and Reverse (ADIPOQ276R AGATGCAGCAAAGCCAAAGT). Primers for ADIPOQ SNP –11391G/A were as follows: Forward GTTGGTGCTGGCATCCTAAG and Reverse GCCTGGAGAACTGGAAGCTG. Each reaction was verified on 2 % agarose gel. As molecular weight marker was used DNA pUC19, hydrolyzed by endonuclease MspI [27].
Serum lipid metabolism parameters (triglycerides (TG), non-esterified fatty acids (NEFA), total and lipoprotein profile cholesterol) and ALT/AST were measured spectrophotometrically. There were estimated plasma total adiponectin (Biovendor, Chezh Republic) and insulin (DRG, Germany) by ELISA. IR was assessed using homeostasis model assessment (HOMA-IR) algorithm [28] and as adipose IR (Adipo-IR, NEFA × insulin) [29]. Unpaired Student’s t test, χ2-test and Spearman’s rank order were used. Data are expressed as mean ± SEM. To predict the probabilities of genetic risk in NAFLD the odds ratio (OR) and 95% confidence interval (CI) were calculated [30].
Results
Comparing with control subjects T2D patients were characterized by overweight and obesity, which were more pronounced at the presence of NAFLD (p < 0.01). It was accompanied by more pronounced increase in НОМА-IR (p < 0.05), TG (p < 0.001), and LDL (p < 0.1) levels. The adiponectin level in women with T2D and NAFLD was
5.92 ± 0.66 mg/ml (10.3–14.5 mg/ml in controls), and in men
it was 4.71 ± 0.54 mg/ml (7.0–10.5 mg/ml in controls); non-NAFLD women with T2D had 4.32 ± 0.50 mg/ml, and male — 4.09 ± 0.51 mg/ml. In both groups, adiponectin levels were significantly lower (p < 0.001) than in control individuals (15.23 ± 0.63 mg/ml).
We found, that Adipo-IR was higher in T2D (164.32 ± 13.23 mmol/L·pmol/L) as compared to controls (62.35 ± 9.36 mmol/L·pmol/L, p < 0.001), and this index was significantly increased in T2D patients with NAFLD as compared to non-NAFLD diabetic subjects (190.18 ± 22.15 vs 133.32 ± 13.58 mmol/L·pmol/L, p < 0.02). Interestingly, there was a correlation between Adipo-IR and adiponectin level in T2D patients with NAFLD only (rs = –0.350, p = 0.021; rs = 0.051, p = 0.762 in non-NAFLD diabetics).
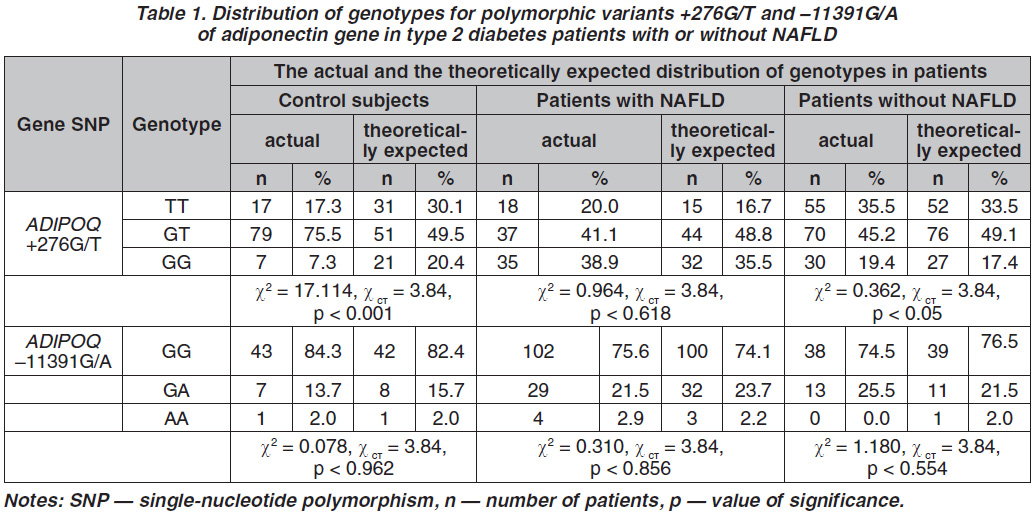
The genotype and allele frequencies of ADIPOQ SNP +276G/Т and –11391G/A are shown in Table 1. For SNP +276G/T 155 patients with T2D without NAFLD and 90 T2D patients with NAFLD were genotyped. Stratification of non-NAFLD patients by +276G/T genotype suggests the prevalence of heterozygotes GT and homozygotes TT compared with GG homozygotes. Thus, the rs1501299 G-allele increased the risk of NAFLD in comparison with T-allele (OR = 4.44, 95% CI = 2.89–6.81, p < 0.05).
The frequencies of alleles among healthy persons for polymorphic variants ADIPOQ +276G/T were
pG = 0.45, pT = 0.55. The frequencies of alleles for polymorphic variants ADIPOQ +276G/T among T2D patients without NAFLD were pG = 0.42, pT = 0.58; among the patients with T2D complicated NAFLD were
pG = 0.41, pT = 0.59. No statistically significant diffe–rences in allele frequencies between studied groups were found (p > 0.05).
We also found that there was a significant difference in frequency of ADIPOQ SNP -11391G/A between T2D patients and control subjects, but not between the patients with and without NAFLD.
The ADIPOQ SNP –11391G/A in controls followed Hardy-Weinberg equilibrium (HWE) (χ2 = 0.078,
p = 0.962) but in case of +276G/Т, it was out of HWE (χ2 = 17.114, p < 0.001). The most prevalent genotype for the +276G/Т SNP was GT in both the non-NAFLD T2D patients (45.2 %) and T2D individuals with NAFLD (41.1 %). The most prevalent genotype of the –11391G/A SNP was GG in both the T2D patients with NAFLD (75.6 %) and without NAFLD (74.5 %). As shown in Table 1, genotype frequencies of +276G/Т were significantly higher in the NAFLD group than in the non-NAFLD (χ2 = 10.61, p < 0.005). The genotype frequencies also were significantly different between NAFLD group and control subjects (p < 0.01). We evaluated also the frequencies of ADIPOQ –11391G/A alleles in Kharkiv population and the possibility of their further
use as a prognostic marker for the risk of T2D complica–ted by NAFLD. Frequencies of ADIPOQ –11391G/A alleles for healthy individuals were: pG = 0.911, pA = 0.088. The frequencies of ADIPOQ –11391G/A alleles for total T2Ds were pG = 0.758, pA = 0.134. No significant differences in allele frequencies between studied groups were found (p > 0.05). The distribution of genotypes in the case and control groups corresponds to the HWE. Frequencies of ADIPOQ –11391G/A alleles for T2Ds with NAFLD were pG = 0.863, pA = 0,137. No statistically significant differences of the allele frequencies between the control group and T2Ds with NAFLD were
found (p > 0.05). Also, there were any significant diffe–rences of allele frequencies between T2Ds with or without NAFLD (pG = 0.873, pA = 0.127). Thus, the initial data shown that homo- or heterozygous carriers of allele A (AA + GA) have increased risk of T2D (OR = 1.45, 95% CI 0.60–4.57), and the absence of this allele (GG-genotype) reduces the risk (OR = 0.69, 95% CI 0.29–1.22) as compared to the average for the whole population.
We did also haplotype analysis in order to get more accurate assessment about the influence of the two
ADIPOQ gene polymorphisms.
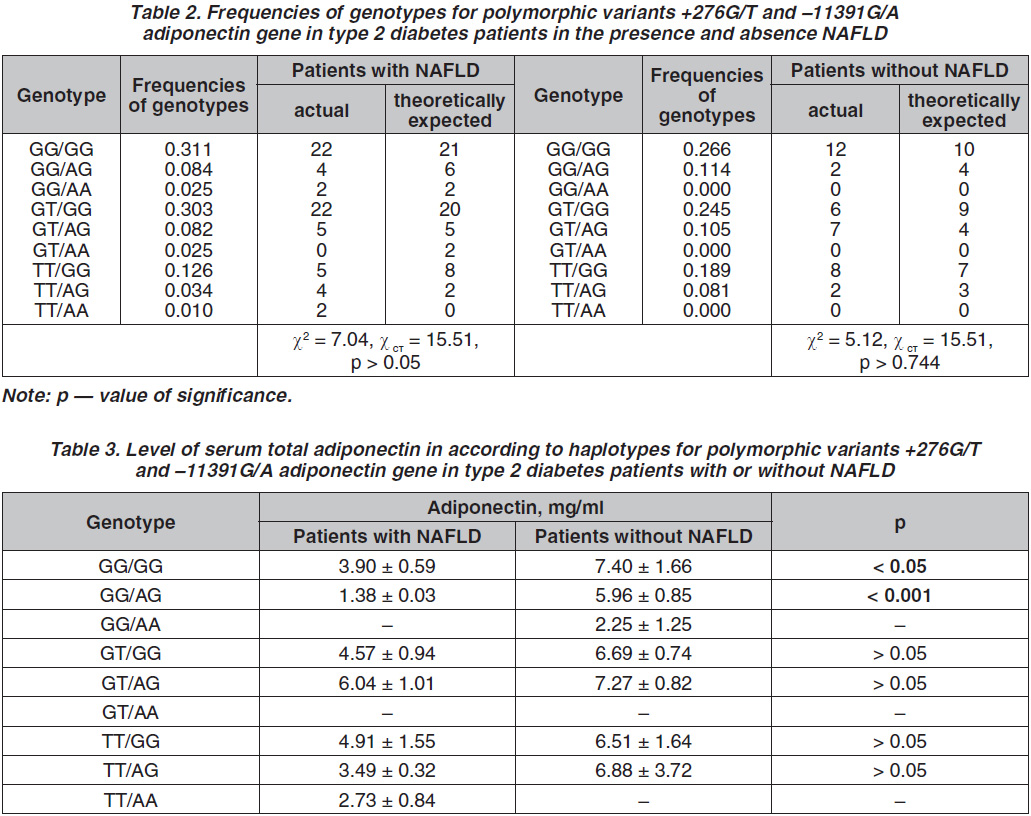
As a result of a haplotypic analysis of the 2 polymorphic variants of the ADIPOQ gene in patients with T2D and NAFLD (table 2), the tendency to increase the frequency of the haplotype GT/GG was shown in comparison with the sample of patients without the liver disease, but the difference was not statistically significant (p > 0.05). It was found that the haplotype of GT/GG was more common in patients with T2D complicated by NAFLD and was twice less often detected in patients without hepatic disease (33 and 16.49 %, respectively, p < 0.05).
According to the results of our study, there was no significant difference in adiponecin levels in blood and frequency distribution of the genotypes of the polymorphic loci G276T and G11391A of the adiponectin gene in patients with T2D with and without NAFLD
(p > 0.05). But in the analysis of haplotypes at these polymorphic loci, it was found that the levels of adiponectin in the group of patients with T2D and NAFLD are numerically or, in some cases statistically, lower than in the group without NAFLD (table 3).
Patients with NAFLD had significantly lower total adiponectin levels than controls (5.32 ± 0.70 vs 15.23 ± 0.63 mg/ml, p < 0.001). In addition, patients with T2D and NAFLD who carried the GG/GG and GG/AG haplotypes of polymorphic variants +276G/T and -11391G/A adiponectin gene had significantly lower serum total adiponectin levels than patients without NAFLD who carried the GG/GG- or GG/AG-genotype. Moreover, in patients with T2D without NAFLD, there was no significant difference in plasma adiponectin levels between patients with different genotypes (p > 0.05).
It was found also, that SNP +276G/Т, but not SNP –11391G/A, had the effect on adipose resistance to insulin in T2D patients with or without NAFLD. Regarding SNP +276G/Т the TT-genotype exhibited numerically higher levels of Adipo-IR-index, which was statistically increased in TT-carriers with BMI ≥ 30 kg/m2 (239.62 ± 36.89 vs 110.54 ± 25.97 mmol/L·pmol/L in T2Ds with BMI < 26 kg/m2, p < 0.05). After the adiponectine gene haplotype analysis we revealed, that this trend (T-allele effect) had been demonstrated only in TT/GG- and GT/GG-carriers with NAFLD (314.56 ± 79,59 vs 144.22 ± 47.12 mmol/L·pmol/L in non-NAFLD T2D TT/GG-carriers, p < 0.02; 135.48 ± 21,88 vs 76.59 ± 15.60 mmol/L·pmol/L in non-NAFLD T2D GT/GG-carriers, p < 0.05) against the background of similarly enhanced (but less pronounced) Adipo-IR in all another genotypes and groups (data not shown).
Discussion
At present time, T2D is considered as a multifactorial, polygenic disease. The level of family risk incompatible with any of the hypotheses of monogenic inheritance, without the additional assumption of incomplete penetrance of the putative gene, which from a formally gene–tic point of view coincides with the polygenic hypothesis, indicates the polygenic nature. A multifactorial model of inheritance suggests that the manifestation of a disease is determined by the interaction of multiple environmental and genetic factors. By genetic factors, it means certain alleles of a number of polymorphic genes involved in the development of T2D, which in clinical practice are called alleles predisposing to the development of T2D. The study of hereditary predisposition to multifactorial diseases is extremely important for their diagnosis and selection of optimal therapy. Thus, of great practical value is the study of polymorphic markers in candidate genes, whose products are involved in the pathogenesis of a multifactorial disease. In this study, we investigated the possible association between ADIPOQ candidate gene polymorphisms of and NAFLD in the group of patients with T2D in Kharkiv population. It was recently shown that the level of fat in the liver correlated with such indicators of total fat as BMI and body fat percent. Furthermore, there is clear correlation between the liver fat accumulation of and WHR [31]. In addition, large population studies have proved an association of nonalcoholic steatohepatitis with increased BMI. It was shown that 91 % of individuals with a BMI over 30 kg/m2 had signs of steatosis by ultrasound data [32].
Adipose tissue IR is present in the majority of patients with NAFLD, whether they are obese or not, and adipose tissue lipolysis provides approximately 60 % of the fatty acids used for hepatic triglyceride synthesis [33, 34]. Even though hepatic IR has been documented in T2D patients using homeostasis model assessment of IR (HOMA-IR) as a measure, there is insufficient data on adipose IR (Adipo-IR) and its relationship with the dysregulation of adipokines in T2D and NAFLD. We first found, that Adipo-IR index was significantly increased in T2D patients with NAFLD as compared to obesity-matched non-NAFLD diabetic subject with pronounced negative correlation between Adipo-IR and adiponectin level in T2D patients with NAFLD only. We found also, that the minor allele of SNP +276G/Т, but not SNP –11391G/A, had the substantial effect on adipose resistance to insulin in T2D patients with NAFLD. Therefore, we can recommend Adipo-IR index as a predictive marker for the NAFLD development and the indicator for monitoring therapy success in T2D patients.
Hipoadiponectinemia considered as unfavorable prognostic sign because adiponectin has angioprotective properties [35]. In NAFLD patients, adiponectin levels are generally decreased [36]. Decreased plasma adiponectin levels were observed in obese and T2D patients, as well as in the ob/ob mouse line (mice with congenital obesity and hyperglycaemia) [37, 38]. In our previous study hipoadiponectinemia was found in all patients with T2D without modulating influence of the NAFLD [39]. This trend is confirmed in the current work, in both diabetic groups, with or without NAFLD, adiponectin le–vels are significantly lower (p < 0.001) than in control subjects. Our results shown, in contrast to previous studies in
the other countries [40, 41], that levels of total adiponectin in circulation were characterized by no gender difference in T2D patients. Moreover, there was no effect of NAFLD on adiponectin levels in T2D patients likely due to the fact that adiponectin is synthesized exclusively by immature adipocytes and depends largely on insulin, glycemia and NEFA levels, which were not statistically different in patients with T2D with or without NAFLD (p > 0,05). On the other hand, we found that total adiponectin levels in patients with T2D and NAFLD who carried the GG/GG- and GG/AG-haplotypes of ADIPOQ SNPs +276G/T and –11391G/A were significantly lower than in T2D patients without NAFLD who carried the GG/GG- or GG/AG-genotype.
Low levels of adiponectin can promote the development of IR by blocking the phosphorylation of insulin receptor, increasing the flow of fatty acids in the liver and slowing their oxidation, thus, improving liver glucose, TG, and LDL production. So far, there are no comprehensive information about genetic determination of fat accumulation in the whole body and in the liver in humans. It is believed that gene polymorphism that exists in patients with NAFLD, associated with a large number of substances involved in the metabolism of lipids and carbohydrates in the liver.
The distribution of genotypes in the control group for ADIPOQ SNP +276G/T, which at some assumptions can be considered as a sample of the population, the ratio deviates from HWE toward high heterozygo–sity. The share of heterozygotes +276G/T at 1.53 times higher (p < 0.001) than the theoretically expected value at equilibrium [42]. Our data correspond in part to the other study which revealed that adiponectin +276 G/T polymorphism was not significantly different between NAFLD and controls, but among females, the GG geno–type was reported to be significantly more prevalent in patients with NAFLD [43].
Genetic variation in ADIPOQ –11391G/A was associated with plasma levels of adiponectin, obesity and resistance to insulin, also tested the association between insulin resistance and –11391G and BMI, suggest that the impact of the variant adiponectin gene polymorphism on glucose homeostasis may depend on the body fat [44]. Due to reduced activity and low circulating levels of adiponectin associated with an increased risk of serious diseases such as T2D and coronary heart disease, experimental data suggest that minor haplotype (by promoter polymorphisms) affects the expression of adiponectin in vivo to such measures that might be unfavorable [45]. SNPs in the promoter and intron 2 regions of ADIPOQ gene play a functional role in adiponectin regulation [46].
G. Dolley et al. (2008) did not found the influence of ADIPOQ gene polymorphisms –11391G/A and –11377C/G on anthropometric indicator associations separately, but haplotype was associated with WHR. If there was a genotype –11391A and 11377C, WHR was significantly higher compared to other haplotypes. Also AC/AC-haplotype carriers had higher levels of adiponectin than the carriers of GG/GG-haplotype (p < 0.0001) [47].
Our data shown that homo- or heterozygous allele A (AA + GA) increases the risk of T2D (OR = 1.45, 95% CI 0.60–4.57), and the absence in the genotype the
A-allele reduced the risk (OR = 0.69, 95% CI 0.29–1.22) compared to the average for the whole population. However, the odds ratio was not statistically significant, perhaps due to the limited amount of samples, which did not allow to achieve the minimum capacity criteria which justifies further studies with increased samples.
Thus, verification of SNPs of adiponectin gene and characteristics of its association with the level of the hormone in the circulation for patients with T2D complicated by NAFLD allow improved preventive therapy algorithm.
NAFLD is a complex disease, which may be the results of multiple loci commonly affected mutations [48, 49]. Previous studies have shown that polymorphisms of adiponectin gene were associated with NAFLD [50]. At the same time, in our study the combination of GG/GG- and GT/GG-haplotypes (33 % respectively) in patients with NAFLD was more frequent. Some combinations of alleles of different loci (haplotypes) can give a highly adapted phenotype, a combination of other alleles give phenotypes poorly adapted. Such deviations from equilibrium may mean that carriers of some genotypes are likely to have a selective advantage, possibly GT/AA have reduced adaptivity, and their absence may be due to the action of selection or the small number of samples.
Conclusions
Our results demonstrate for the first time, that Adipo-IR index was significantly increased in Ukrainian T2D patients with NAFLD as compared to obesity-matched non-NAFLD diabetic subject with pronounced negative correlation between Adipo-IR and adiponectin level in T2D patients with NAFLD only. We grounded the prospects of using the Adipo-IR index for monitoring of T2D therapy and associated liver disease development. Against the background of the research the adiponectin gene polymorphic variant haplotype association with NAFLD in T2Ds in the Kharkiv region population it have been established new markers (rs1501299 G-allele, rs17300539 and rs1501299 GG-/GG- and GT/GG-haplotypes, respectively) of an increased risk of NAFLD development in T2D patients. Thus, the contribution of various factors to the development of the NAFLD is significantly different depending on the population. Further identification of genetic markers for NAFLD risk in patients with T2D will allow to understand the main pathological mechanisms of the diseases, and to select the respectively treatment.
Conflicts of interests. Authors declare the absence of any conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
Information on funding. Financial support: National Academy of Medical Sciences of Ukraine, Ministry of Education and Science of Ukraine, #0108U001147, #0111U000174.
Information on contribution of each author:
Yu. Karachentsev — coordination of the project, within the framework of which the research was conducted, the concept of work;
M. Gorshunska — key participation in data collection, diagnosis of type 2 diabetes mellitus, discussion of the data;
T. Tyzhnenko — creating a control group, analysis of genetic data and their discussion, participation in creating the database, writing the text of the article;
N. Krasova — conducting biochemical studies (carbohydrate/lipid metabolism, assessment of insulin resistance), analysis and discussion, writing the text of the article;
I. Dunaeva — participation in data collection, diagnosis of non-alcoholic fatty liver disease, performing and analyzing anthropometric studies;
A. Gladkih — carrying out immunoassay studies;
Zh. Leshchenko — biochemical studies (lipid metabolism);
A. Pochernyaev — conducting molecular genetic studies, participation in the creation of a database;
T. Mishchenko — search for information and literature sources, participation in the systematization of the material;
O. Plohotnichenko — conducting statistical analysis of the data;
L. Atramentova — coordination of molecular genetic studies, the choice of statistical methods of the research;
N. Kravchun — coordination of the project, within the framework of which the research was conducted, the design of the study, determination of the cohort of patients with non-alcoholic fatty liver disease;
V. Poltorak — the concept of work, the development of the plan and objectives of the study, generalization of the results.
Список литературы
1. Lonardo A. Nonalcoholic fatty liver disease: A precursor
of the metabolic syndrome [Теxt] / A. Lonardo, S. Ballestri,
G. Marchesinic, P. Angulod, P. Loria // Digestive and Liver Di–sease. — 2015. — Vol. 47. — P. 181-190.
2. Williams T. Metabolic Syndrome: Nonalcoholic Fatty Liver Disease [Теxt] / T. Williams // FP Essent. — 2015. — Vol. 435. — P. 24-29.
3. Nonalcoholic fatty liver disease as a feature of the metabolic syndrome [Теxt] / P. Socha, A.Wierzbicka, J. Neuhoff-Murawska et al. // Rocz Panstw Zakl Hig. — 2007. — Vol. 58(1). —
P. 129-137.
4. Fotbolcu H. Nonalcoholic fatty liver disease as a multi-systemic disease [Теxt] / H. Fotbolcu, E. Zorlu // World J. Gastroenterol. — 2016. — Vol. 22(16). — P. 4079-4090.
5. Association between hypoadiponectinemia and cardiovascular risk factors in nonobese healthy adults [Теxt] / J.A. Im, S.H. Kim, J.W. Lee et al. // Metabolism. — 2006. — Vol. 55,
№ 11. — P. 15 46-1550.
6. Chen W. The relationship between nonalcoholic fatty liver and insulin resistance with abnormal glucose metabolism [Теxt] / W. Chen, Z. Yu, Y. Li // Zhonghua Gan Zang Bing Za Zhi. — 2000. — Vol. 8. — P. 76-77.
7. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome [Теxt] / G. Marchesini, M. Brizi, G. Bianchi et al. // Diabetes. — 2001. — Vol. 50. — P. 1844-1850.
8. Sharabi Y. Nonalcoholic fatty liver disease is associated with hyperlipidemia and obesity [Теxt] / Y. Sharabi, A. Eldad // American Journal of Medicine. — 2000. — Vol. 109. — P. 171.
9. Li Y.Y. Genetic and epigenetic variants influencing the development of nonalcoholic fatty liver disease [Text] /
Y.Y. Li // World J. Gastroenterol. — 2012. — Vol. 18(45). —
P. 6546-6551.
10. Fotbolcu H. Nonalcoholic fatty liver disease as a multi-systemic disease [Text] / H. Fotbolcu, E. Zorlu // World J. Gastroenterol. — 2016. — Vol. 22(16). — P. 4079-4090.
11. Adiponectin, an adipocyte-derived plasma protein, inhi–bits endothelial NF-kappaB signaling through a cAMP-dependent pathway [Теxt] / N. Ouchi , S. Kihara, Y. Arita et al. // Circulation. — 2000. — Vol. 102, № 11. — P. 1296-1301.
12. Association of adiponectin gene variations with risk of incident myocardial infarction and ischemic stroke: a nested case-control study [Теxt] / H.H. Hegener, I.M. Lee, N.R. Cook et al. // Clin Chem. — 2006. — Vol. 52(11). — P. 2021-2027.
13. Adiponectin and C-reactive protein in obesity, type
2 diabetes, and monodrug therapy [Text] / D.M. Putz, W.S. Goldner, R.S. Bar et al. // Metabolism. — 2004. — Vol. 53. — P. 1454-14561.
14. Association of the +33371 A/G polymorphism in adiponectin receptor 2 gene with Type 2 diabetes in the Chinese population [Теxt] / Y.F. Liao, L.L. Chen,T.S. Zeng et al. // J. Endocrinol. Invest. — 2007. — Vol. 30, № 10. — P. 860-864.
15. Mendez-Sanchez N. Adiponectin, structure, function and pathophysiological implications in non-alcoholic fatty liver disease [Теxt] / N. Mendez-Sanchez, N.C. Chavez-Tapia, D. Zamoravaldes, M. Uribe // Mini Rev. Med. Chem. — 2006. — Vol. 6(6). —
P. 651-656.
16. Gene polymorphisms associated with nonalcoholic fatty liver disease and coronary artery disease: a concise review [Теxt] / Xiao-Lin Li, Jian-Qing Sui, Lin-Lin Lu [et al.] // Lipids in Health and Disease. — 2016. — Vol.15. — P.1-8.
17. Obesity-induced DNA hypermethylation of the adiponectin gene mediates insulin resistance [Text] / A.Y. Kim, Y.J. Park, X. Pan et al. // Nat. Commun. — 2015. — Vol. 6. —
P. 7585.-8585.
18. Regulation of gelatin-binding protein 28 (GBP28) gene expression by C/EBP [Text] / K. Saito, T. Tobe, M. Yoda et al. // Biol. Pharm. Bull. — 1999. — Vol. 22(11). — P. 1158-1162.
19. Genomic structure and mutations in adipose-specific gene, adiponectin [Text] / M. Takahashi, Y. Arita, K. Yamagata et al. // Int. J. Obes. Relat. Metabol. Disord. — 2000. — Vol. 24(7). — P. 861-868.
20. Genomewide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21-q24 [Теxt] / N. Vionnet, E.H. Hani, S. Dupont et al. // Amer. J. Hum. Genet. — 2000. — Vol. 67(6). — P. 1470-1480.
21. Genome-wide search for type 2 diabetes in Japanese affec–ted sib-pairs confirms susceptibility genes on 3q, 15q, and 20q and identifies two new candidate Loci on 7p and 11p [Теxt] / Y. Mori, S. Otabe, C. Dina et al. // Diabetes. — 2002. — Vol. 51(4). — P. 1247-1255.
22. The genes influencing adiponectin levels also influence risk factors for metabolic syndrome and type 2 diabetes [Теxt] / A.G. Comuzzie, M.E. Tejero, T. Funahashi et al. // Hum. Biol. — 2007. — Vol. 79(2). — P. 191-200.
23. A haplotype at the adiponectin locus is associated with obesity and other features of the insulin resistance syndrome [Теxt] /
C. Menzaghi, T. Ercolino, R.D. Paola et al. // Diabetes. — 2002. —
Vol. 51. — Р. 2306-2312.
24. Однонуклеотидний поліморфізм гена адипонектину (+276>Т) та експресія складових інсулінорезистентного стану у хворих на цукровий діабет 2 типу [Текст] / М.Ю. Горшунська, Ю.І. Караченцев, Н.О. Кравчун та ін. // Проблеми ендокринної патології. — 2013. — № 2. — С. 7-17.
25. Q192R polymorphism of PON-1 gene in type 2 diabetes patients [Теxt] / M.Yu. Gorshunskaya, Yu.I. Karachentsev, L.A. Atramentova et al. // Cytology and Genetics. — 2011. — Vol. 45(1). — P. 38-40.
26. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroentero–logy, and the American Gastroenterological Association [Теxt] / N. Chalasani, Z. Younossi, J.E. Lavine et al. // Hepatology. — 2012. — Vol. 55(6). — P. 2005-2023.
27. Гендерные различия и генетический полиморфизм адипонектина у детей с алиментарным ожирением [Текст] / А.В. Солнцева, Е.А. Аксенова, А.В. Сукало и др. // Известия национальной академии наук Беларуси. — 2011. — № 2. — С. 29-37.
28. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man [Теxt] / D.R. Matthews, J.P. Hoske, A.S. Rudensk et al. // Diabetologia. — 1985. — Vol. 28. — P. 412-419.
29. Adams-Huet B. Increased adipose tissue insulin resistance in metabolic syndrome: relationship to circulating adipokines [Text] / B. Adams-Huet, S. Devaraj, D. Siegel, I. Jialal // Metab. Syndr. Relat. Disord. — 2014. — Vol. 12(10). — P. 503-507.
30. Armitage P. Statistical methods in medical research [Text] / P. Armitage, G. Berry. — 3rd ed. — Blackwell Scientific Publications, 1994. — 620 p.
31. Body fat distribution and insulin resistance: beyond obesity in nonalcoholic fatty liver disease among overweight men [Text] / S.H. Park, B.I. Kim, S.H. Kim et al. // Journal of the American College of Nutrition. — 2007. — Vol. 26(4). — Р. 321-326.
32. Взаємозв’язок неалкогольної жирової хвороби печінки та розвитку ускладнень у хворих на цукровий діабет 2 типу, терапевтичні підходи (огляд літератури) [Текст] / Н.О. Кравчун, В.В. Полторак, О.В. Земляніцина // Проблеми ендокринної патології. — 2011. — № 1. — C. 67-75.
33. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms [Теxt] / E. Bugianesi, A. Gastaldelli, E. Vanni et al. // Diabetologia. — 2005. —
Vol. 48. — P. 634-642.
34. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease [Теxt] / K.L. Donnelly, C.I. Smith, S.J. Schwarzenberg et al. // J. Clin. Invest. — 2005. — Vol. 115. — P. 1343-1351.
35. Hypertrophic Mesenteric Adipose Tissue May Play a Role in Atherogenesis in Inflammatory Bowel Diseases [Теxt] /
E. Theocharidou, A. Balaska, K. Vogiatzis et al. // Inflamm. Bowel. Dis. — 2016. — Vol. 22(9). — P. 2206-2212.
36. Buechler C. Adiponectin, a key adipokine in obesity related liver diseases [Теxt] / C. Buechler, J. Wanninger, M. Neumeier // World J. Gastroenterol. — 2011. — Vol. 7(23). — P. 2801-2811.
37. Plasma concentrations of a novel, adiposespecific protein, adiponectin, in type 2 diabetic patients [Теxt] / K. Hotta, T. Funahashi, Y. Arita et al. // Arteriosclerosis, thrombosis, and vascular biology. — 2000. — Vol. 20(6). — P. 1595-1599.
38. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice [Теxt] / A. Xu, Y. Wang, H. Keshaw et al. // J. Clin. Invest. — 2003. — Vol. 112. — P. 91-100.
39. Однонуклеотидний полiморфiзм –11391 G>A гена адипонектину (ADIPOQ) у хворих на цукровий дiабет 2 типу, ускладнений неалкогольною жировою хворобою печiнки [Текст] / Т.В. Тижненко, Л.О. Атраментова, Ю.I. Караченцев та ін. // Проблеми ендокринної патології. — 2014. — № 3. — С. 7-17.
40. Fibrosis is associated with adiponectin resistance in chronic hepatitis C virus infection [Теxt] / S. Corbetta, A. Redaelli, M. Pozzi et al. // Eur. J. Clin. Invest. — 2011. — Vol. 41. — P. 898-905.
41. Noreldin N. Low Serum adiponectin correlates with liver fibrosis in patients with chronic hepatitis c infection [Text] / N. Noreldin, M.M. Shareef // Journal of American Science. — 2014. — Vol. 10(9) — P. 36-40.
42. Гетерозиготность по SNP +276 G>T гена адипонектина как потенциальный предиктор устойчивости к сахарному диабету 2 типа [Текст] / Ю.И. Караченцев, М.Ю. Горшунская, Л.А. Атраментова и др. // Актуальні проблеми акушерства і гінекології, клінічної імунології та медичної генетики. — Луганськ, 2010. — С. 195-199.
43. Influence of adiponectin gene polymorphisms in Japanese patients with non-alcoholic fatty liver disease [Text] / K. Toku–shige, E. Hashimoto, H. Noto et al. // J. Gastroenterol. — 2009. — Vol. 44(9). — P. 976-982.
44. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice [Теxt] / J. Fruebis, T.S. Tsao, S. Javorschi et al. // Proc. Natl Acad. Sci. USA. — 2001. — Vol. 98(4). — P. 2005-2010.
45. Haplotypes in the promoter region of the ADIPOQ gene are associated with increased diabetes risk in a German Caucasian population [Теxt] / P.E. Schwarz, S. Govindarajalu, W. Towers et al. // Horm. Metab. Res. — 2006. — Vol. 38. — P. 447-451.
46. Harvest F. Gu Biomarkers of Adiponectin: Plasma Protein Variation and Genomic DNA Polymorphisms [Теxt] / F. Gu Harvest // Biomarker Insights. — 2009. — Vol. 4. — P. 123-133.
47. Promoter adiponectin polymorphisms and waist/hip ratio variation in a prospective French adults study [Теxt] / G. Dolley, S. Bertrais, V. Frochot et al. // Int. J. Obes. (Lond). — 2008. — Vol. 32(4). — P. 669-675.
48. Puppala J., Bhrugumalla S., Kumar A. et al. Apolipoprotein C3 gene polymorphisms in Southern Indian patients with nonalcoholic fatty liver disease // Indian J. Gastroenterol. — 2014. — Vol. 33. — P. 524-529.
49. Sookoian S., Castaño G.O., Scian R. et al. Genetic variation in transmembrane 6 superfamily member 2 and the risk of nonalcoholic fatty liver disease and histological disease severity // Hepatology. — 2015. — Vol. 61. — P. 515-25.
50. AdipoQ T45 G and G276 T polymorphisms and susceptibility to nonalcoholic fatty liver disease among asian populations: a meta-analysis and meta-regression [Теxt] / B.F. Wang, Y. Wang, R. Ao et al. // J. Clin. Lab. Anal. — 2016. — Vol. 30(1). — P. 47-57.