The suffix “omics” refers to fields that study a particular class of molecules, such as gene, proteins, and metabolites, focusing on their function and relationship [1]. Omics disciplines include genomics, epigenomics, transcriptomics, proteomics, metabolomics, lipidomics, and nutrigenomics, among many others [2].
Genomics aims to evaluate the structure of an organism’s genome, including mapping genes and DNA sequencing, examining the molecular mechanisms and provide information related to genetic profiles, its influence on organs and systems, and the interaction of genetic factors in various diseases. Functional genomics, another field of molecular biology, studies the regulation of a gene expression and its effects on protein translation and protein-protein interactions [3, 4]. Moving from genomics to transcriptomics, the interest moves from genes to the RNA transcript (the trascriptome), studying its changes due to alterations in the genome, its variabi–lity among different cells, and responses to different biological conditions or pharmacological treatment. Mirro–ring the term genomics, proteomics investigates protein function, post-translational modifications, and interaction while metabolomics aims to identify and quantify small molecules that aremetabolic products of different biological and pathological process.
From genomics to metabolomics, such technologies have progressed considerably in recent years, not only in basic research, but they are also becoming more readi–ly used as a source of precious information that can be easily transferable into clinics in the form of diagnostic tests. However, one of the greatest obstacles that omics technologies must overcome is related to sample procurement. In order, for findings from omics studies to be translated into clinics, sufficient numbers of samples need to be obtained in order for the findings to be sufficiently reliable and robust. Thus, it is imperative that up-to date information is shared within the clinical field, improving the general understanding of the technologies and promoting stronger collaborations between research and clinics.
1. Introduction to proteomics
Proteomics is the omics field that deals with screening the entire collection of proteins within a cell. This complete collection of proteins is known as the “proteome”. The study provides information regarding protein abundances, their variations and modifications, along with their interacting partners and networks, in order to understand cellular processes. Another important aspect is that proteins are unstable and dynamic in contrast to the relatively static genome. The localization, number, and concentration of proteins may change depending on the ongoing condition and disease. “Clinical proteomics” is a subdiscipline of proteomics that involves the application of proteomic technologies on clinical specimens such as blood or urine.
There are many arguments that highlight the necessity for non-invasive biomarkers that could detect the early onset of kidney disease, monitor responses to the–rapy, and predict progression to end-stage renal disease. With regards to the study of CKD, urinary proteomics/peptidomics arguably represents the most appropriate approach. This can be rationalized by the fact that the proteins present in urine are more likely to be a reflection of the pathological state of the organ. Mischak et al. insist that such biomarkers could substantially improve patient management, providing prognostic information and predicting responses to treatment, guiding the choice of therapy. By all means, omics are going to improve the understanding of kidney disease at a molecular level and provide the potential to identify superior therapeutic targets that can improve patient care [5].
Despite diverse challenges, proteomics that employs bodily fluids is thought to be one of the most promising approaches for detecting disease biomarkers. Furthermore, the identification of novel therapeutic treatments is another promising area in which proteomics is applied. Going beyond this, proteomics is also starting to play a significant role in the validation of drug targets, understanding the mechanism of drug action, drug metabolism, and drug toxicity [6].
2. Advancements in analytical techniques
The field of proteomics has rapidly developed and has been involved in numerous research areas, by employing a vast array of modern analytical techniques. Initially, two-dimensional gel electrophoresis (2DE) was employed. While although technical issues related to inter-gel variability were partially overcome by the development of two-dimensional differential gel electrophoresis (2D-DIGE), the large step forward came when liquid chromatography (LC) was coupled with mass spectro–metry (MS), enabling untargeted protein identification. In LC-MS, analytes are separated within the LC column prior to electrospray ionization (ESI) and detection in a highly specific and sensitive manner. As a result, LC-MS has been employed for protein biomarker discovery –using a wide array of biological fluids. Furthermore, metho–dologies employing capillary electrophoresis (CE)-MS have also developed very rapidly during recent years, –owing to its ability to rapidly separate analytes in a highly reproducible manner. In CE-MS, proteins, or peptides, are separated based upon their migration time through a capillary prior to MS detection. Despite CE-MS not being particularly suited for the analysis of larger proteins (> 20 kDa), it has become readily used for the analysis of the urinary peptidome due to its compatibility with high salt concentrations [7]. As a result of these develo–ping technologies, both targeted and non-targeted protein identification became available even with a small sample volumes. Furthermore, information related to post-translational modifications can now be obtained, witch such modifications playing a significant role du–ring di–sease development [6, 8]. The potential of applying these mass spectrometric approaches to biological fluids means that they have evolved to become readily employed in a wide variety of different clinical studies [9].
With regards to the analysis of tissue, the ability to detect changes in protein expression directly in situ represents a very appealing aspect with regards to the detection of specific protein markers. Modern mass spectrometry imaging (MSI) techniques, particularly Matrix-assisted laser desorption/ionization (MALDI), have developed rapidly in recent years and are now able to analyse tissue specimens with sufficient spatial resolution in order to distinguish individual cells, pushing the boundaries even further. The application of this technique directly on renal tissue can provide detailed information related to renal diseases and the potential to detect specific protein markers. Therefore, the article will focus more specifically on this rapidly emerging technology and provide li–terature examples of how MALDI-MSI has already been applied in the study of CKD.
2.1. MALDI-MSI
MALDI and MSI appeared when in 1985 F. Hillenkamp and M. Karas first used this technology to analyse proteins, peptides, sugars, and polymers which ionized in a “soft” manner to create single-charged ions [10]. Taking into account the ability of MALDI to analyse proteins and numerous other different substances and its’ widespread availability, it remains the most commonly applied MSI technique. As the proteins play a significant role in the wide array of pathways involved in cellular signalling cascades, the ability to resolve their spatial localization concurrently within the same section of tissue can significantly facilitate the detection of pathological processes and hence determine the risk-groups. It’s also increasingly common used for the detection of lipids and metabolites, meaning that multiple disease mechanisms can be described and integrated with proteomic fin–dings. Since the development of MALDI-MSI, it has employed in a large number of clinically orientated studies, focusing on fields such as oncology, pathology, and even forensics [11]. It also proved to be a valuable tool for the detection of xenobiotics and their metabolites directly in situ [12].
Generally speaking, MALDI-MSI is performed by acquiring a mass spectrum at specific spatial coordinates within a defined measurement region, which is usually related to an entire section or particular regions of inte–rest present within a tissue section. Using the acquired mass spectra, the spatial distribution of the analytes (metabolites, lipids, proteins) can be visualised and a molecular image of the tissue reconstructed. These molecular images can then be correlated with tissue images obtained traditional histology.
Specific steps of specimen preparation include tissue washing in order to remove potential contaminants (eg. salt and polymers), on-tissue digestion with protease enzymes (commonly trypsin), and finally matrix application itself [13, 14]. Fresh samples are considered to be the primary source of tissue for MALDI-MSI experiments and are commonly collected for this type of analysis. However, fresh samples also need to be frozen immediately following collection in order to stabilise the proteome through inhibition of the enzymatic processes. The significant advantage offered by fresh-frozen (FF) tissue is that it closely mimics the native state of the tissue, with the tissue morphology and integrity being preserved. The freezing process here must be performed in a gradual and homogenous manner in order to avoid the formation of ice crystals, which can lead to tissue cra–cking. The most common approach involves loosely wrapping the tissue in aluminium foil and freezing in li–quid nitrogen or cooled alcohol (to approximately –70 °С for approximately one minute. Alternatively, the tissue can also be cooled in isopentane dry ice. A further approach that can be employed in order to avoid protein degradation is conductive heat transfer. However, it is important to verify the compatibility of each tissue with this treatment, given that the tissue morphology can be altered during the process. Once stabilisation has been performed, the tissue is stored at –80 oС prior to –MALDI-MSI analysis.
In recent years, which is also arguably of greater significance to clinical MALDI-MSI studies, protocols have been developed in order to enable formalin-fixed paraffin-embedded (FFPE) tissue to be analysed [13]. FFPE tissue represents the major proportion of the patient samples collected and stored in hospitals and other medical centres, meaning that they are becoming invaluable to histopathological studies involving MALDI-MSI and can unearth a plethora of new information. Ultimately, the analysis of FFPE tissue enables retrospective studies with much larger cohorts of patients. This is especially important when attempting to collect samples of particularly rare diseases, which would take a consi–derably longer period of time if attempting to obtain an equivalent number of FF specimens. In terms of sample storage, FFPE tissue can also be stocked at room temperature for up to 10 years, far greater than would be possible with FF tissue, even at –80 oС.
In general, MALDI-MSI has vast applicability in the study of various diseases, and these will be briefly touched upon prior to discussing its relevance in CKD.
Of particular note, Baluff et al. decided to employ MALDI-MSI in order to study different tumour microenvironments in gastric cancer and breast carcinoma. Using this approach, they were able to identify diffe–rent tumour microenvironments within tumour tissue that appeared homogenous when evaluated using traditional histological techniques. Employing a novel combination of segmentation and multivariate analysis methods, they detected a number of tumour subpopulations, within histologically homogenous regions, that were associated with changes in the levels of DEFA-1 and Histone H2A. This molecular information was then combined with clinical data taken from patients in order to predict the survival rate of patients based upon the number of observed molecular tumour sub-populations. This is a prime example of not only how MALDI-MSI can provide information that is confirmatory, or complimentary to histological information, but also how it can provide further information that was not previously possible [15, 16].
Y. Ucal et al. also provided an extensive summary of the role MALDI-MSI in various other cancer types and neurodegenerative diseases. Using MALDI-MSI, proteomic signatures of different grades of glial tumors, specific peptides in multiple myeloma, the distribution of pharmacological agents in ovarian cancer, changes in glycosylation patterns in prostate cancer, and the scree–ning of renal cell carcinoma have all been performed. Furthermore, specific proteins implicated in Alzhei–mer’s, Parkinson’s, and Huntington’s disease have also been obtained and represent highly promising, if not initial, studies [9, 17].
3. Proteomics applications in CKD
The scope to analyse a wide variety of diseases –using modern proteomic techniques is vast, however, we have to keep in mind that chronic kidney disease (CKD) is one of the major health and socioeconomic burdens. Thus, there is a strong need for new reliable diagnostic biomarkers and markers indicative of treatment efficacy that can be used alongside the classical clinical and pathological tools. The world nephrology and pathology community still continues to struggle in detecting the disease onset, predicting the individual’s risk of disease development and/or progression, prediction to therapy response, and classification. It is already known that proteomics could be a reliable diagnostic method itself [18, 19] given that the large proportion of urinary proteins are generated by the kidney and are thus carrying the substantial information about renal state [18].
Chronic glomerulonephritis is one of the major causes of CKD and there are a number of existing studies that focus on primary and secondary glomerulonephritis, focusing on focal segmental glomerulosclerosis (FSGS), IgA-nephropathy, membranous glomerulonephritis (MGN) and minimal change disease (MCD) [19]. Initially, several studies have employed animal models in order to study these renal diseases. Xu et al. employed laser capture microdissection (LCM) to specifically isolate glomeruli from rats with FSGS, with the glomerular proteome then being analysed by MALDI-MS. Proteomic patterns of sclerotic and nonsclerotic glomeruli within FSGS were generated. However, they also noted that non-sclerotic glomeruli within FSGS were in fact more similar to sclerotic glomeruli than completely normal glomeruli from a proteomic standpoint, hypothesizing that the early onset of sclerotic processes can be detected by molecular analyses at an earlier stage [20]. Employing a mouse model, Kaneko et al. studied the pathogenesis of IgA nephropathy (IgAN). The authors investigated the molecular distribution of a variety of lipids in IgA murine kidneys using MALDI-MSI and noted that there were a number that were over-expressed in cortical regions of kidneys affected by the disease, with respect to healthy controls [21].
Mainini et al. first investigated the potential of employing MALDI-MSI directly on human renal bioptic material. It was noted that the glomeruli and tubules of healthy kidney tissue present similar proteomic profiles. However, in cases of primary glomerulonephritis (GN), including MGN and MCD, proteomic differences were observed between glomeruli and tubules. Furthermore, proteomic alterations were observed in GN tubules, even in regions without morphological indications of the disease. This finding indicated that it is possible to detect pathological alterations at the molecular level prior before being able to note changes with traditional histology [22].
Building upon this, Smith et al. applied MALDI-MSI to bioptic renal tissue taken from patients with the most frequent glomerular kidney diseases: FSGS, IgAN and membranous nephropathy (MN). They succeeded in generating molecular signatures capable of discriminating normal from GN tissue, as well as detecting a number of potential molecular markers of CKD progression. More specifically, they also detected a number of signals specific for each form of GN. One particular protein, identified as alpha-1-antitrypsin (A1AT) by MS/MS, was shown to be localised to the podocytes of sclerotic glomeruli following antibody validation. Given the localization of this protein, they hypothesized that its presence may be associated with podocyte stress that occurs during the development of sclerosis. Furthermore, the same peptide fragment of A1AT was detected in the urine of GN patients who were shown to progress to the latter stages of renal disease. This body of work highligh–ted how findings generated by MALDI-MSI could also be also translated into less-invasive proteomic analysis employing urine.
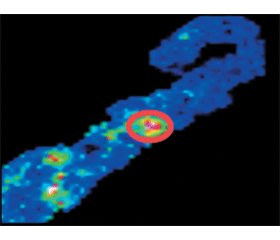
MALDI-MSI has also been recently employed in the study of membranous nephropathy (MN) which is the leading cause of nephrotic syndrome in adults and one of the most leading nephropathies worldwide. As this disease can manifest as both primary (idiopathic) or secon–dary, it was important to find the relevant biomarkers to distinguish these two forms, considering that it is not always possible to do this using clinical information alone, as this enables the most appropriate treatment and management to be selected. The work of Beck et al. presented a large step forward in the diagnosis of MN, detecting the circulating antigens phospholipase A2 receptor (PLA2R), IgG4, and thrombospondin type 1 domain-containing 7A (THSD7A) in the sera of primary MN patients [23]. However, there is still the strong need for further markers that can be used to stratify primary MN patients, given that the aforementioned antigens only account for approximately 75 % of primary MN patients. Here, Smith et al. employed MALDI-MSI to FFPE tissue of 20 patients to evaluate the capability of this technology to detect alterations in the tissue proteome of primary and secondary MN patients and represented the first example of MALDI-MSI being applied to FFPE renal biopsies for this purpose. The positive results obtained with this proteomic approach facilitated the detection of a number of signals that could differentiate the different forms of iMN that were positive to PLA2R or IgG4 as well as distinguish primary from secondary MN. In particular, MALDI-MSI was able to generate molecular signatures of primary and secondary MN, with one particular signal (m/z 1459), identified as Serine/threonine-protein kinase MRCK gamma, being over-expressed in the glomeruli of primary MN patients with respect to secondary MN. Furthermore, this proteomic approach detected a number of signals that could differentiate the different forms of iMN that were positive to PLA2R or IgG4 as well as a further set of signals (m/z 1094, 1116, 1381 and 1459) that distinguish these patients from those who were ne–gative to both. Although this study is only in the initial phase, the preliminary results are encouraging and the line of work holds a fair degree of promise. Following verification on a larger cohort of patients, the signals detected here could potentially represent future proteomic markers of iMN [24].
/43-1.gif)
All these studies show vast potential and represent the value of employing modern proteomic techniques for diagnostic purposes, particularly in CKD. It is hoped that this already strong platform will encourage further, more in-depth, studies that could eventually reveal new specific and sensitive biomarkers that could be used for the diagnosis, prognosis, and management of renal disease. Renal diseases, as a very specific entity, have been more widely investigated and understood than ever before during recent decades. However, gi–ven the ever increasing worldwide incidence of CKD, in addition to advancements in analytical instrumentation, it is both important, and possible, to obtain much more molecular information than was ever possible. Understanding the molecular nature of this disease will open new horizons in its diagnosis management stra–tegies.
A vast amount of information on this topic is accessible on the OMICS international website which includes open-access journals and other various information (https://www.omicsonline.org/) as well as the CKD database (http://www.padb.org/ckddb/).
Conflicts of interests: not declared.
Список литературы
1. Horgan RP, Kenny LC. ‘Omic’ technologies: genomics, transcriptomics, proteomics and metabolomics. The Obstetrician & Gy–naecologist 2011;13:189–195.
2. Mina H. Hanna & Alessandra DallaGassa&Gert Mayer &GianluigiZaza& Patrick D. Brophy& Loreto Gesualdo& Francesco Pesce. (2016) The nephrologist of tomorrow: towards a kidney-omic future. PediatrNephrol DOI 10.1007/s00467-016-3357-x
3. Pesce F, Pathan S, Schena FP (2013) From -omics to perso–nalized medicine in nephrology: integration is the key. Nephrol Dial Transplant 28:24–28
4. Holtorf, Hauke; Guitton, Marie-Christine; Reski, Ralf (2002). "Plant functional genomics". Naturwissenschaften. 89: 235–249. doi:10.1007/s00114-002-0321-3
5. Mischak H, Delles C, Vlahou A, Vanholder R (2015) Proteomic biomarkers in kidney disease: issues in development and implementation. Nat Rev Nephrol 11:221–232
6. Yan Shi-Kai, Liu Run-Hui, Jin Hui-Zi, Liu Xin-Ru, Ye Ji, Shan Lei, Zhang Wei-Dong. Omics in pharmaceutical research: overview, applications, challenges, and future perspectives Chinese Journal of Natural Medicines 2015, 13(1): 0003–0021
7. Albalat, A., Franke, J., Gonzalez, J., Mischak, H., Zürbig, P., Urinary proteomics based on capillary electrophoresis coupled to mass spectrometry in kidney disease. Methods Mol. Biol. Clifton NJ 2013, 919, 203–213.
8. Hu S, Loo JA, Wong DT (2006) Human body fluid proteome analysis. Proteomics 6:6326–6353
9. Kasap M, Akpinar G, Kanli A. Proteomic studies associated with Parkinson's disease. Expert Rev Proteomics. 2017 Feb 4. doi: 10.1080/14789450.2017.1291344. [Epub ahead of print]
10. Karas M., Bachmann D., Hillenkampf F. (1985) Influence of the Wavelight in High-Irradiance Ultraviolet Laser Desorption Mass Spectrometry of Organic Molecules”. Anal. Chem. 57 (14): 2935-9.
11. Chughtai K, Heeren RMA. Mass spectrometric imaging for biomedical tissue analysis.
12. Cornett DS, Frappier SL, Caprioli RM. MALDI-FTICR imaging mass spectrometry of drugs and metabolites in tissue. Anal Chem. 2008;80(14):5648-5653.doi:10.1021/ac800617s. Chem Rev. 2010;110(5):3237-3277.
14. Gabriele De Sio, Andrew James Smith, Manuel Galli, MattiaGarancini, CliziaChinello, Francesca Bono, Fabio Pagni and FulvioMagni (2015) A MALDI–Mass Spectrometry Imaging method applicable to different formalin-fixed paraffin-embedded human tissues. DOI: 10.1039/c4mb00716f
15. Ruben D. Addie, Benjamin Balluff, Judith V.M.G. Bovee, Hans Morreau and Liam A. McDonnell. Current State and Future Challenges of Mass Spectrometry Imaging for Clinical Research Anal. Chem. Anal. Chem., 2015, 87 (13), pp 6426–6433 DOI:10.1021/acs.analchem.5b00416
16. Benjamin Balluff, Christian K Frese, Stefan K Maier, CedrikSchoene, Bernhard Kuster, Manfred Schmitt, MichaelaAubele, Heinz Hofler, Andre M Deelder, Albert JR Heck, Pancras CW Hogendoorn, Johannes Morreau, AF Maarten Altelaar, Axel Walch and Liam A McDonnell. De novo discovery of phenotypic intratumor heterogeneity using ima–ging mass spectrometry. J Pathol 2015; 235: 3-13. DOI: 10.1002/path.4436
17. Deininger SO, Ebert MP, Futterer A, et al. MALDI imaging combined with hierarchical clustering as a new tool for the interpretation of complex human cancers. J Proteome Res 2008; 7: 5230–5236.
18. Y. Ucal, et al., Clinical applications of MALDI imaging technologies in cancer and neurodegenerative diseases, Biochim.Biophys. Acta (2017), http://dx.doi.org/10.1016/j.bbapap.2017.01.005
19. Sun L., Zou L. — X. Chen M. — J. Make precision medicine work for chronic kidney disease. Free supplementary material. Med PrincPractDOI:10.1159/000455101
20. Smith A, L’Imperio V, De Sio G, et al. Alpha‑1-Antitrypsin detected by MALDI imaging in the study of glomerulonephritis: Its relevance in chronic kidney disease progression. Proteomics. 2016. Jun;16(11-12):1759- 66. DOI 10.1002/pmic.201500411
21. Xu BJ, Shyr Y, Liang X, et al. Proteomic patterns and prediction of glomerulosclerosis and its mechanisms. J Am SocNephrol. 2005;16(10):2967-2975.
22. Kaneko Y, Obata Y, Nishino T, et al. Imaging mass spectro–metry analysis reveals an altered lipid distribution pattern in the tubular areas of hyper-IgA murine kidneys. Exp MolPathol. 2011;91(2):614-621.
23. Veronica Mainini, Fabio Pagni, Franco Ferrario, Federico Pieruzzi, Marco Grasso, Andrea Stella, Giorgio Cattoretti &FulvioMagni. MALDI imaging mass spectrometry in glomerulonephritis: feasibility study. Histopathology 2014, 64, 901–906. DOI: 10.1111/his.12337
24. Beck LH, Bonegio RGB, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11-21. doi:10.1056/NEJMoa0810457.
25. Andrew Smith, Vincenzo L’Imperio, Elena Ajello, Franco Ferrario, NiccoloMosele, Martina Stella, Manuel Galli, CliziaChinello, Federico Pieruzzi, Goce Spasovski, Fabio Pagni, FulvioMagni, The putative role of MALDI–MSI in the study of Membranous Nephropathy, BBA — Proteins and Proteomics (2016), doi: 10.1016/j.bbapap.2016.11.013

/43-1.gif)