The article was published on p. 91-95
Introduction
Bicarbonate-based hemodialysis (BHD) relies on the on-line preparation of the dialysate from a concentrated acidic electrolyte solution which is diluted and mixed with bicarbonate solution to achieve usual final concentrations dictated by pre-set conductivity and bicarbonate targets. Acidic electrolyte solution contains acetic acid (in most western countries to a final concentration of 3 mM) to stabilize the solution and avoid calcium and magnesium salts precipitation, mostly as carbonates. Despite this, circuit scale remains a problem with dialysis machines, requiring frequent descaling [1]. When mixed with bicarbonate, acetic acid reacts with bicarbonate to give acetate and carbonic acid (i.e. CO2), to a final partial pressure of about 97 mmHg [2]. Dialysate CO2 freely diffuses through the filter membrane to the patient blood, resulting in significant load to the patient. Lung ventilation easily removes this CO2 load preventing pCO2 to rise in arterial blood. In patients with marginal lung function the BHD-related CO2 load may result in some degree of CO2 body retention and clinical consequences.
We describe a case of intradialytic symptomatic hypercapnia in a patient with respiratory insufficiency; acetate-free biofiltration (AFB) allowed successive uneventful dialysis treatments.
Case presentation:
This 82-year old female patient was transferred in our Nephrology Unit because of «acute on chronic» renal failure and anuria. She had known chronic renal insufficiency with a serum creatinine of 2 mg/dL, obesity, hypertension, hypercholesterolemia, gout, diverticulosis, hypokinetic dilated cardiomyopathy with FE 36 % and was in NYHA class 2B classification. She had been admitted to the emergency room several days ago because of acute pulmonary congestion and high ventricular response atrial flutter; she was at first treated with Continuous Positive Airway Pressure (CPAP) and diuretics. She later underwent a coronary angiography and angioplasty with everolimus-medicated stenting of a critical proximal circumflex stenosis. Because of multiple alternating episodes of paroxysmal atrial fibrillation and bradycardia she also had a bicameral pace maker implanted. Lastly she developed severe sepsis, worsening myocardial function and oliguria, and was treated with continuous venous-venous hemofiltration (CVVH).
After patient stabilization, respiratory support changed to O2 supplementation by open mask and intermittent hemodialysis was then considered. Shortly after the first dialysis treatment start, she developed worsening dyspnea. An arterial blood gas analysis showed respiratory acidosis with a pH of 7,25, pCO2 48.1 mmHg, pO2 56.2 mmHg, lactate 3.6 mmmol/l and bicarbonate 23.8 mmol/l. Despite high volume O2 through the mask the patient didn’t get better, and the session was stopped with rapid resolution of symptoms.
The day after a new gas analysis (on 4 l/min O2 through mask) showed arterial pH 7.36, pCO2 42 mmHg, pO2 106 mmHg, sO2 98 %, bicarbonate 24.4 mmol/L, lactate 0.3 mmol/l. A chest radiography showed bilateral pleural effusions and raised diaphragm.
We considered that dialysis-induced CO2 load was responsible for the acute and reversible episode of respiratory acidosis of the previous day, and decided to treat the patient with AFB, with serial controls of acid-base parameters (Table 2). We used post-dilution bicarbonate infusion with a 145 mM concentration, with infusion rate aimed at a final bicarbonate concentration of 23 mM according to manufacturer’s algorithm. The run was conducted uneventfully, as were several additional successive treatments. Lastly she resumed diuresis with stable renal function and serum creatinine at 2.1–2.3 mg/dl.
She still needed 2–4 l/min O2 to keep sO2 at 96–98 % with a paCO2 of 29 mmHg, with persistent pleural medium-basal effusion on the left.
Discussion
Peculiar changes of acid-base parameters occur during a BHD session, representing instant blood/dialysate equilibration within the dialyzer, intradialytic overall base balance and respiratory accommodation [3–5].
As summarized in Table 1, the dialysate is more acidic than blood, with a pH ranging from 7.1 to 7.3 and a partial pressure of CO2 approximating 70–100mmHg.
H2CO3/CO2 originates in small part from the concentrated bicarbonate solution, and mostly from the chemical reaction between acetic acid (the stabilizing and acidifying agent in the concentrated electrolyte solution, necessary as already said to prevent Ca and Mg salts precipitation) and bicarbonate. In aqueous solution, pCO2(mmHg) is about [H2CO3] (mM)/0.0309; since 3 mmol/l of bicarbonate react with acetic acid, this corresponds to a final concentration in the dialysate of 3 mM acetate (which accounts for a fraction of positive base balance to the patient) and carbonic acid (which dissociates into H2O and CO2, resulting in calculated pCO2 of 97 mmHg [2]; actual measured values are somehow lower, representing escape from the solution through degassing devices in the circuit.
CO2 has a high solubility and diffusibility, rapidly equilibrating with patient’s blood flowing through the dialyzer and increasing pCO2 in outlet blood to the patient. The increase in pCO2 is significantly higher than the bicarbonate rise, which is 4 times less diffusible through the membrane than CO2. A gas analysis carried out in inlet blood (representing patient’s arterial systemic blood if an A-V fistula is in use) will show metabolic acidosis, but in the filter bicarbonate, acetate, CO2 and oxygen are taken up so that outlet blood will show respiratory acidosis without hypoxia [6] Patients with physiological lung function are able to excrete dialysis-related CO2 load during the first blood pass through the lungs, so that in arterial (pre-filter) blood pCO2 is no longer increased, or only slightly so [7].
Table 2 summarizes the changes in acid-base parameters occurring in the dialysis circuit (inlet vs outlet, dialysate and blood) in a spot BHD time, as well as the prospective changes in systemic (pre-filter) blood along the dialysis session in a representative patient.
In quantitative terms dialysis-associated CO2 load to patients amounts to about 60 mmol/hour, about 10% of endogenous metabolic load [8]; accordingly, it is estimated that an increase of about 10% of the pulmonary ventilation is necessary for the disposal of this CO2 load. In occasional patients with impaired respiratory reserve, CO2 retention and respiratory acidosis may develop in the course of BHD [3]. Patients with chronic lung disease start BHD with higher levels of pCO2 and lower pO2 than healthy controls, and achieve higher pCO2 and lower pO2 during the first hour of treatment [7]. With higher dialysate acetate concentration (4 or 5 mM), respiratory difficulties are known to occur even more frequently [1]. While slowly developing hypercapnia is usually well tolerated by the body, acute hypercapnia may have serious adverse consequences on heart function and rhythm, coronary flow past a critical stenosis, mental status (with both agitation and depression of consciousness), pulmonary function (pulmonary vasoconstriction and ventilation/perfusion mismatch) and cell metabolism [9]. It is of note that our patients, as well as a similar case [10], was acutely symptomatic a short time after the beginning of dialysis, indicating that the rapidity in change, rather than absolute pCO2 level, was responsible for symptoms. Discontinuation of dialysis and associated CO2 load ra-pidly restores clinical condition [10, 11].
To overcome the risk of CO2 overload in patients with reduced respiratory reserve needing dialysis alternative modalities to traditional BHD are to be sought; since dialysate is the source of CO2 load, one might envisage as a first approach to reduce dialysate flow to less than the traditional 500 ml/min, i.e. to about 200–300 ml/min. No published data concerning gas and pulmonary changes during a low-volume dialysate exist, to our knowledge; reduced efficiency (in terms of quantitative waste solute removal) of such a procedure has to be anticipated, requiring longer or more frequent sessions [12]. An alternative choice might be acetate-based hemodialysis (i.e. without bicarbonate), which is associated with CO2 loss through the dialyzer [13]. However this technique also induces profound pulmonary hypoventilation with intradialytic hypoxia; additionally, acetate-based dialysate is almost unavailable today from the market. It should be noted that substituting acetic acid with other acidifying compounds (e.g. citric acid, as in current use, at a final 1 mM concentration) in BHD does not result in less CO2 generation, since the same amount of HCO3- reacts with the acid (which dissociates 3 protons).
Finally, a different approach to the CO2 problem is AFB. This type of hemodialysis uses a completely buffer-free dialysate and relies in the direct post-dilution (post-filter) infusion of isotonic bicarbonate for correction of acidosis [14, 15]. It is a diffusion/convection-based methodology, whereby bicarbonate losses and convective fluxes in the dialyzer are matched by post-dilution bicarbonate reinfusion; convection fluxes and reinfusion rates are modeled in order that a progressive rise of positive bicarbonate balance and of systemic bicarbonate blood levels induce a progressive increase of bicarbonate loss in the dialyzer until a pre-defined equilibrium between infusion and losses is reached, with stable bicarbonate systemic blood levels. Table 1 summarizes composition of dialysate in BHD and AFB, and of bicarbonate solution for reinfusion in AFB; it can be seen that almost no CO2 is present in the AFB dialysate, which in fact takes up CO2 (and bicarbonate) from the patient (about 15–20 mmol/hour CO2; see dialysate out, Table 3A).
/94.jpg)
This is much less than CO2/H2CO3 infused with the bicarbonate solution (about 4–6 mmol/hour); since pH of bicarbonate reinfusion solution is higher than in patient’s blood, some H2CO3/CO2 may be formed by chemical reaction of bicarbonate with weak acids in blood (e.g. monobasic phosphate), in a quantity hard to calculate, possibly not higher than a few mmoles along the whole treatment time. Thus infused CO2 remains far less than CO2 lost through the dialyzer, and actually pCO2 slightly falls in systemic blood during AFB. Table 3 summarizes the changes in acid-base parameters occurring in dialysate and blood along the dialysis circuit in a «spot» AFB time, as well as the prospective changes in systemic (pre-filter) blood along an AFB session in a representative patient. One should note that in this bicarbonate and acetate-free dialysate electroneutrality is maintained by high Cl– concentration; this does not result in hyperchloremia because bicarbonate reinfusion (at an almost «physiological» Na+ concentration) dilutes plasma anions of the same magnitude that it increases bicarbonate concentration. The manufacturer provides a simple electronic program or tables to set reinfusion and convection fluxes according to the bicarbonate bag in use (of 3 available: 120, 145 and 167 mM, the 145 mM being the most used), and pre-set final bicarbonate and Na+ concentrations.
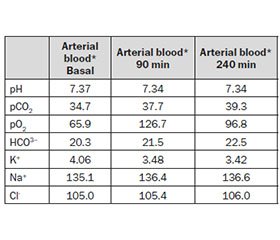
In our patient we modeled a «safe» final bicarbonate level of 23 mM, but more convenient levels of 28–30 mM are usually chosen in standard patients. Table 4 summarizes acid-base and electrolyte changes in the presented patient’s arterial blood at different points of treatment: as can be seen, paCO2 remained stable, target bicarbonate level was achieved, and no hypoxia occurred.
Take home message: BHD is associated with a small, but significant CO2 load to the patient; in patients with reduced pulmonary reserve (for acute or chronic conditions), this load may be associated with acute rise in systemic blood pCO2 and acute symptoms of respiratory distress. AFB avoids to load patients with CO2 (actually it removes it) and does not negatively impact on gas blood gases regulation in the course of a dialysis session.
Список литературы
1. Ryzlewicz T. The wrong prescription of dialysis fluid // J. Nephrol. Ther. 2015; 5: e113 doi: 10.4172/2161-0959.1000e113
2. Golper T.A., Fissel R., Fissel W.H., Hartle M., Sanders M.L., Schulman G. Hemodialysis: core curriculum 2014 // Am. J. Kidney Dis. 2014; 63: 153-63.
3. Symreng T., Flanigan M.J., Lim V.S. Ventilatory and metabolic changes during high efficiency hemodialysis // Kidney Int. 1992; 41: 1064-1069.
4. Feriani M. Behaviour of acid-base control with different dialysis schedules // Nephrol. Dial. Transplant. 1998; 13 (S6): s62-s65.
5. Ledebo I. Acid base correction and convective dialysis therapies // Nephrol. Dial. Transplant 2000; 15: 45-48.
6. Marano M. Alterazioni gasanalitiche in bicarbonato dialisi // G. Ital. Nefrol. 2013; 30: 1-8.
7. Alfakir M., Moammar M. Pulmonary gas exchange during hemodialysis: a comparison of subjects with and without COPD on bicarbonate hemodialysis // Ann. Clin. Lab. Science 2011; 41: 315-320.
8. Kao C.C., Guntupalli K.K., Bandi V., Jahoor F. Whole-body CO2 production as an index of the metabolic response to sepsis // Shock 2009; 32: 23-28.
9. Hyz R.C., Hidalgo J. Permissive hypercapnia. UpToDate. Waltham, MA, 2015.
10. Patriarca A., Marano M. Insolita indicazione per l’ AFB: il paziente ipercapnico. Abst 54°congress Italian Society of Nephrologya // G. Ital. Nefrol. 2013; 30 (S61): s88.
11. Marano M., Patriarca A., Zamboli P. Acidosi respiratoria: colpa della fistola arterovenosa. Abst. 54th congress Italian Society of Nephrology // G. Ital. Nefrol. 2013; 30(S61): s88.
12. Sigdell J.E, Tersteegen B. Clearance of a dialyzer under varying operating conditions // Artif. Organs. 1986; 10: 219-225
13. Pitcher W.D., Diamond S. Pulmonary gas exchange during dialysis in patients with obstructive lung disease // Chest. 1989; 96: 1136-1141
14. Martello M., Di Luca M. Acetate Free Biofiltration // G. Ital. Nefrol. 2012; 29 (S55): s62-s71
15. Marano M., D’Amato A., Patriarca A., Di Nuzzi L.M., Giordano G., Iulianiello G. Carbon dioxide and acetate free biofiltration: a relationship to be investigated // Artif. Organs. 2015; doi: 10.1111/aor.12477
16. Roscoe J.M., Goldstein M.B., Halperin M.L., Wilson D.R., Sinebaugh B.J. Lithium-induced impairment of urine acidification // Kidney Int. 1976; 7: 344-350.
/94_2.jpg)

/93.jpg)
/92.jpg)
/94.jpg)