Журнал «Здоровье ребенка» 2 (61) 2015
Вернуться к номеру
Impact of Genetically Predisposed Skin Barrier Function Abnormalities on the Onset and Course of Food Allergy in Children
Авторы: Pakholchuk O.P. — Zaporizhia state medical university, Ukraine
Рубрики: Педиатрия/Неонатология
Разделы: Клинические исследования
Версия для печати
The increase in the incidence of the food allergy (FA) occurs more rapidly than changes in the genome sequence. To investigate the impact of genetically predisposed skin barrier function abnormalities associated with filaggrin (FLG) genes polymorphism on the FA onset and course, we studied 53 children with skin FA symptoms. Genotyping was performed for FLG variants R501X and 2282del4. Results. The frequency of 2 variants in the study population was 0.018 for R501X and 0.075 for 2282del4. The combined allele frequency was 0.096. All patients were heterozygotes. Children with mutations showed significant odds ratio (OR) of 31.3 (95% CI 2.1; 452.0) in terms of early onset of the skin allergy symptoms — during 1st month of life. And had no influence on the FA clinical signs start after 12 months of age (OR 0.5 (0.05; 4.85), p < 0.05). Null mutations were clinically important risk factors for the persistent transepidermal water loss (TEWL). Conclusion. Genetically predisposed skin barrier function abnormalities have impact on early FA onset and are important for persistent TEWL.
Количество пациентов с пищевой аллергией (ПА) растет быстрее, чем меняется геном. С целью изучить влияние генетически обусловленных функциональных аномалий кожного барьера, ассоциированных с мутацией гена филаггрина, на формирование и течение пищевой аллергии в исследование были включены 53 ребенка с кожными проявлениями ПА. Генотипирование было проведено для ноль-вариантов — R501X и 2282del4. Результаты. Частота выявления R501X составила 0,018, а 2282del4 — 0,075, их комбинации — 0,096. Все пациенты были гетерозиготы. Вероятность развития ПА у детей на первом месяце жизни при наличии мутации составила: OR 31,3; 95% CI 2,1; 452,0. Но генетические факторы не влияли на развитие ПА после года (OR 0,5 (0,05; 4,85), p < 0,05). Нулевые мутации были значимыми для персистирующей трансэпидермальной потери жидкости. Выводы. Генетически обусловленные функциональные аномалии кожного барьера влияют на раннее развитие ПА у детей и связаны с уровнем трансэпидермальной потери жидкости.
Кількість пацієнтів із харчовою алергією (ХА) росте швидше, ніж змінюється геном. З метою вивчення впливу генетично обумовлених функціональних аномалій шкірного бар’єра, асоційованих із мутацією гена філагрину, на формування та перебіг харчової алергії у дослідження були включені 53 дитини із шкірними проявами ХА. Генотипування було проведено для ноль-варіантів — R501X та 2282del4. Результати. Частота виявлення R501X становила 0,018, а 2282del4 — 0,075, їх комбінації — 0,096. Усі пацієнти були гетерозиготи. Вірогідність розвитку ХА в дітей на першому місяці життя при наявності мутацій становила: OR 31,3; 95% CI 2,1; 452,0. Однак генетичні фактори не впливали на розвиток ХА після року (OR 0,5 (0,05; 4,85), p < 0,05). Нульові мутації були значимі для персистуючої транcепідермальної втрати рідини. Висновки. Генетично обумовлені функціональні аномалії шкірного бар’єра впливають на ранній розвиток ХА в дітей та пов’язані з рівнем трансепідермальної втрати рідини.
food allergy, filaggrin, gene mutations, children.
пищевая аллергия, филаггрин, генные мутации, дети.
харчова алергія, філагрин, генні мутації, діти.
Статья опубликована на с. 19-22
Food allergy is the most common disease in children and has prevalence of 10–15 % in the preschool age [5]. In some patients it has tendency to spontaneous recovery, but anyway this pathology has big social-economic importance because it is usually chronic [18]. Atopic dermatitis is the most common skin disease in children. But answer on the question «FA via AD»: are they comorbidities or are they manifestation of the same disease?» is still opened. Atopic dermatitis and food allergy are frequently co-exist, particularly in those with early onset, severe and persistent atopic eczema [11]. In practice not all patients with AD has clinically significant FA, even in some of them cause of exacerbation could not be found [18]. On the other hand, not all patients with FA in future develop AD. Last Food Allergy and Anaphylaxis Guidelines underline that AD is the only one (IgE- and cell-mediated) of the clinical manifestations of the FA [5]. Some clinicians consider atopic eczema to be strongly related to food allergy and can be controlled by food avoidance, but others substantially deny such a relationship, limiting investigations for food allergy to severe cases in infancy who do not respond to treatment [19].
Since Hanifin and Rajka proposed Diagnostic Criteria for AD it’s diagnostics is not difficult. But food allergy is only minor criterion. Even opinions based on the given low frequency of food allergies actually inducing flares of AD appeared to return focus to appropriate skin therapy in AD patients. It was reported that identification of true food allergies is necessary only in about 30% of them [10, 18].
It is well accepted that genetic and environmental factors play major roles in the pathogenesis of most diseases. The rising of incidence of the FA occurs more rapidly than changes in the genome sequence. This suggests that the risk of FA is likely to be at least partially environmentally determined [20]. It was shown that frequency of the FA in several childhood periods is different. About two thirds of patients with FA after 5 y.o. have more mild symptoms than in the first years of life [5]. So are all of them genetically predisposed? Hereditary and acquired alterations of structural proteins and lipids of the stratum corneum and epidermal tight junctions leading to a diminished skin barrier function are major causative factors for a number of skin diseases and AD is one of them [17]. Strong association with genetics and pathology was proved only for AD and ichthyosis. Variants on three candidate genes (SPINK5, KLK7 and FLG) have been associated with atopic dermatitis. Filaggrin (FLG) loss-of-function skin barrier gene mutations are associated with atopic dermatitis (AD) and transepidermal water loss (TEWL) [6].
Epidemiological research on comorbidities of skin diseases is increasingly recognized as an important tool to understand their etiologies more fully. Some authors reported a cross-sectional analysis on the complex associations among atopic dermatitis, filaggrin loss-of-function mutations, skin barrier function, and food sensitization. Presence of food sensitization and allergy earlier in life predicts a prognosis of severe AD, but conclusions about its role in the pathogenesis of AD cannot be made. Of note, the strong association between AD and food allergies does not exist in adults with moderate-tosevere AD [1, 10]. Better understanding of the food allergy patho-aetiology will help in development of novel therapeutic interventions and methods of disease prevention.
The aim of the study was to evaluate the impact of the genetically predisposed skin barrier function abnormalities associated with FLG genes polymorphism in the FA onset and course in children. To assess how allele variants influence on the FA course.
Materials and methods
Patients were recruited during 2011–2014 at the allergologic and outpatient departments of the Zaporizhia city multifield children hospital № 5, Zaporizhia, Ukraine and University Hospital of the Zaporizhia state medical university, Zaporizhia, Ukraine. The study was approved by the local ethics committee, conducted according to Declaration of Helsinki principles, and all subjects gave their informed consent. All the children were diagnosed with FA and/or AD by the same pediatrician, based on clinical examination and according to the MOH of Ukraine Diagnostic Criteria, EAACI guidelines [5]. Each child’s skin was examined and the disease severity of eczema score were evaluated using SCORAD scale. Transepidermal water lost (TEWL) was estimated by humidity level, that was measured with skin humidity meter Qeentone, France. Subjects were classified as having comorbidity: allergic asthma or allergic rhinoconjunctivitis based on physician’s diagnosis. 73 % (n = 38) of the patients had onset of food allergy skin symptoms < 1 year of age and the mean age of onset was 6 (3; 12) months.
Total serum-IgE level was evaluated with ELISA. The phenotype «increased total IgE» was analyzed as a qualitative variable, with age–specific cut-off values. The cut-offs were; 22.3 kU/l (9 months — 5 years), 263 kU/l (5–20 years) and 122 kU/l (> 20 years). Deep history analysis was done to identify causative product — cause of FA skin symptoms. They were reported as identified or «difficulties in product identification».
Filaggrin is an essential component in the process leading to the formation of a fully functional skin barrier, responsible for the keratin cytoskeleton and squames formation. Recent studies have identified 2 loss-of-function variants, R501X and 2282del4, in the filaggrin gene as predisposing factors in the development of eczema. [13]. Genomic DNA was extracted from peripheral venous blood using a standard protocol in the Institute of Hereditary Pathology, NAMS of Ukraine, Lviv, Ukraine. Genotyping was performed for FLG — variants R501X and 2282del4 on all 53 individuals.
Statistical analysis was performed using standard commercial software Statistica (Statsoft, USA). All continuous variables were tested for a normal distribution using the Shapiro-Wilk’s W test. Continuous variables are presented as mean ± standard deviation or median (inter-quartile range) if non-normally distributed. Categorical variables are presented as counts and proportions. Odds ratios (OR) estimates including 95% confidence interval (CI) were analysed with Nonlinear estimation, quick Logit regression.
Results
We’ve performed genotyping for FLG variants R501X and 2282del4 on all 53 individuals. The next 2 variants frequency in the study population was 0.018 in the case of R501X and 0.075 for 2282del4. The combined allele frequency was 0.096. None homozygotes for R501X 2282del4 were found. All patients were heterozygotes (fig. 1).
/14/14.jpg)
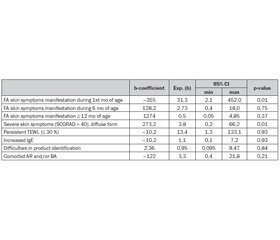
FLG gene mutations influence on the onset and course of the skin food allergy symptoms influence was assessed with Nonlinear estimation with quick Logit regression (table 1). Where exponential level of the b coefficient is odds ratio of each factor. P value showed statistical validity of the given associations.
Children with mutations showed an OR of 31,3 (95% CI 2,1; 452,0) for the skin allergy symptoms early onset: during 1st mo of age, 2,73 (0,4; 18,0) — from birth to 6 mo. And had no influence on the FA clinical signs start after 12 mo of age (OR 0,5 (0,05; 4,85)). Confidence interval of the given odds didn’t cross 1 only for 1st mo of age. This gives opportunity to suggest that gene have influence on the food allergy clinical symptoms onset only in newborns. This fact corresponds to the given in the literature data about environmental impact in the allergy spread over the world. In the case of FA food can play this part.
As it is seen from the table, null mutations were clinically important risk factors for the persistent low skin humidity, that indicates continuous TEWL. This suggests that skin barrier function is important in the persistence of FA, as it was supposed by Tan H.-T. et al., 2014 [14].
In addition to the analysis of associations heterozygotes with FA skin symptoms as frequently as patients with wild type had increased IgE and such comorbidities as AR and BA. Trying to answer the question: «are environmental factors more likely to affect those genetically at risk?», history assessment was done. It showed that children with mutations had no problems with FA symptoms cause product identification and could name it. Similar data were given by Flohr C. et al. (2014) that studied only 3 mo exclusively breastfed infants and indicated that FLG mutations were not significantly associated with food sensitization in children with AD (OR = 1.21, 0.45–3.24, p = 0.70) [4].
Discussion. Allergic reactions to foods are an important medical problem. FA occurrence seems to be strongly influenced by genetics, but the basis of the genetic predisposition to food allergy has not been differentiated from that for atopy in Ukraine. Our results are in concordance with other authors data. Swedish study showed almost the same separate and combined allele frequency [12]. This fact underline that Ukrainian children can be referred to European population and probably they have the same patterns. Anyway the FLG gene mutation frequency is little in children with FA. And it corresponds to the common conclusion that the rising incidence is occurring more rapidly than changes to the genome sequence would allow [20]. Relationships of FLG null mutations with other allergic diseases such as bronchial asthma and allergic rhinitis also have been reported. However, the role of common variants of FLG in atopic eczema and other allergic diseases is poorly understood [12]. Our study results underline that FLG gene mutations are associated with FA not as with AR or BA. This corresponds with other authors data [15]. Moreover other authors studies facilitate the development of early subgroup-specific interventions to prevent the progression from eczema, associated with FA, to asthma.
Association of the null mutations only with early FA onset phenotype and clinical importance of the persistent skin barrier dysfunction that was showed in our study gives us opportunity to suggest influence of the FLG genetically brake on the older children through TEWL. And the latest study results partially confirmed this hypothesis [3, 14].
On the other hand it was shown that skin inflammation lead to the skin barrier deficiency and TEWL appears secondary. Epigenetics has recently been considered as a potential mechanism involved in the development of many disorders, including allergic diseases. Animal models have shown that environmental factors such as maternal tobacco smoke or mechanical ventilation can alter gene transcription and consequently the structure and function of lungs. In fact, the exposure to environmental factors during early childhood may induce a long-lasting altered genetic state adapting to a persistent «Th2 state» thus influencing the development of asthma or atopic dermatitis and food allergy if alterations involve the filaggrin gene [6]. The lack of filaggrin expression observed in the analysed corneal specimens from AD patients is not due to the two most common FLG mutations (R501X, 2282del4) but is most likely secondary to inflammation, as all keratitis specimens of non-AD patients showed lack of filaggrin expression as well [2]. These indicate that other than FLG gene mutations factors can downregulate profilaggrin/filaggrin expression. Literature review showed that Venkataraman D. et al. detected no direct effect of FLG mutations on FA at any age; however, an indirect effect was found on FA at all ages through eczema and FA symptoms in the earlier years [9].
Further perspectives. Determination of the mechanisms of the epigenetic environmental factors action on the FA onset and it interaction with genetic risk should be provided. Since FLG expression was identified on the mucosa cells further studies of the FLG null mutations and gut permeability are needed [7].
Conclusion. Genetically predisposed skin barrier function abnormalities presence alone does not explain the prevalence of food allergy. It have impact in early FA onset and is important for persistent TEWL. Environmental influences in the food allergy onset and course in children is underestimated.
1. Alternative models of comorbidity: a framework for the interpretation of epidemiological association studies / J. Schmitt, S. Weidinger // J. Invest. Dermatol. — 2014. — № 134(2). — P. 303–7.
2. Analysis of filaggrin mutations and expression in corneal specimens from patients with or without atopic dermatitis / T. Lapp, C. Auw-Haedrich, T. Reinhard [et al.] // Int. Arch. Allergy Immunol. — 2014. — 163(1). — P. 20–4.
3. Application of moisturizer to neonates prevents development of atopic dermatitis / K. Horimukai, K. Morita, M. Narita [et al.] // J. Allergy Clin. Immunol. — 2014. — № 134(4). — P. 824–830.
4. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants / C. Flohr, M. Perkin, K Logan [et al.] // J. Invest. Dermatol. — 2014. — № 134(2). — P. 345–50.
5. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy / A. Muraro, T. Werfel, K. Hoffmann–Sommergruber [et al.] // Allergy. — 2014. — № 69(8). — P. 1008–25.
6. Epigenetics of allergy / G. Tezza, F. Mazzei, A. Boner // Early Hum. Dev. — 2013. — № 89, Suppl 1. — P. 20–1.
7. Filaggrin haploinsufficiency is highly penetrant and is associated with increased severity of eczema: further delineation of the skin phenotype in a prospective epidemiological study of 792 school children / S.J. Brown, C.L. Relton, H. Liao [et al.] // Br. J. Dermatol. — 2009. — № 161(4). — P. 884–889.
8. Filaggrin is expressed in the epithelial cells of the buccal mucosae / M. Sebbag, G. Serre and M. Simon // Pediatric Allergy and Immunology. — 2014. — Vol. 25, Issue 6. — P. 600–601.
9. Filaggrin loss-of-function mutations are associated with food allergy in childhood and adolescence / D. Venkataraman, N. Soto-Ramírez, R.J. Kurukulaaratchy [et al.] // J. Allergy Clin. Immunol. — 2014. — № 134(4). — P. 876–882.
10. Food Allergy and Atopic Dermatitis: Separating Fact from Fiction / K.-Y. Suh // Semin. Cutan. Med. Surg. — 2010. — № 29. — P. 72–78.
11. Food allergy and atopic eczema / A. Worth, A. Sheikh // Curr. Opin. Allergy Clin. Immunol. — 2010. — № 10(3). — P. 226–30.
12. Food sensitization in Japanese infants is associated with a common Filaggrin variant / T. Nomura, I. Tsuge, C. Inuo [et al.] // Ann. Allergy Asthma Immunol. — 2013. — № 110(5). — P. 388–390.
13. Loss-of-function Variants of the Filaggrin Gene are Associated with Atopic Eczema and Associated Phenotypes in Swedish Families / E. Ekelund, A. Lied én, J. Link [et al.] // Acta Derm. Venereol. — 2008. — № 88. — P. 15–19.
14. Methylation of the filaggrin gene promoter does not affect gene expression and allergy / H.-T.T. Tan, J.A. Ellis, J.J. Koplin [et al.] // Pediatric Allergy and Immunology. — 2014. — Vol. 25, Issue 6. — P. 608–610.
15. No filaggrin gene mutation in a patient with a combination of atopic dermatitis, alopecia areata and food allergy / S. Ono, A. Otsuka, Y. Miyachi, K. Kabashima [et al.] // Eur. J. Dermatol. — 2012. — № 22(6). — P. 785–6 (doi: 10.1684/ejd.2012.1875).
16. No remarkable differences in rates of sensitization to common type I and IV allergens between FLG loss-of-function mutation carriers and wild-type subjects / L. Landeck, M. Visser, C. Skudlik [et al.] // Contact Dermatitis. — 2014. — № 70(1). — P. 27–34.
17. Skin barrier in atopic dermatitis / S. Kezic, N. Novak, I. Jakasa [et al.] // Front. Biosci. (Landmark Ed). — 2014. — № 1; 19. — P. 542–56.
18. The Diagnosis and Graded Therapy of Atopic Dermatitis / T. Werfel, N. Schwerk, G. Hansen, A. Kapp // Dtsch. Arztebl. Int. — 2014. — № 111. — P. 509–20.
19. The role of food allergy in atopic dermatitis / M. Greenhawt // Allergy Asthma Proc. — 2010. — № 31(5). — P. 392–7.
20. The role of genetics and environment in the rise of childhood food allergy / T.H.-T. Tan, J.A. Ellis, R. Saffery, K.J. Allen // Clinical & Experimental Allergy. — 2012. — № (42). — P. 20–29.

/14/14_2.jpg)